
January 2020
with addendum


© Crown copyright 2020
This publication is licensed under the terms of the Open Government Licence v3.0 except where otherwise stated. To view this licence, visit nationalarchives.gov.uk/doc/open-government-licence/version/3
Where we have identified any third party copyright information you will need to obtain permission from the copyright holders concerned.
This publication is available at www.infectedbloodinquiry.org.uk
Any enquiries regarding this publication should be sent to us [email protected]
01/20
Printed on paper containing 75% recycled fibre content minimum
Printed in the UK by the APS Group
This is the first of two documents compiled by the clinical group in response to the questions posed in the Letters of Instruction of September and December 2019.* The document is set out in a way that follows a progression through the natural history, diagnosis and treatment of HIV, which means that the points are not always in the same numerical order and supplementary points are inserted where they appear to fit best. The numerical identifier of each point is clearly identified within the text. Because certain questions in the Letters of Instruction may have areas of overlap, the text of some responses has been duplicated across sections.
*The second document answering the remaining questions is attached as an addendum
The document is written with reference to the situation in the UK. The British HIV Association (BHIVA) has developed an extensive library of evidence-based guidelines and standards which are the quality benchmark for diagnosis, treatment and care for people living with and affected by HIV in the UK. There is also national guidance from, among others, the British Association of Sexual Health and HIV (BASHH), Public Health England (PHE), NHS England and the National Institute for Health and Care Excellence (NICE). These are key sources that are frequently cited within this report.
The terminology and language used throughout this document aim to be consistent with the UNAIDS (2015)1 recommendations.
HIV (human immunodeficiency virus) is a virus that preferentially attacks and kills the particular cells (the CD4 T lymphocytes) that control the immune system and thereby allow the body to fight infection. By damaging the immune system, HIV makes a person vulnerable to life-threatening infections and cancers. If left untreated, HIV can lead to the condition AIDS (acquired immune deficiency syndrome). HIV is the cause of one of humanity's most serious and persistent epidemics. Since the first reports of AIDS in 1981, more than 78 million people are estimated to have been infected with HIV and 39 million people have died. At least 36.9 million people worldwide are living with HIV infection. Today, with the knowledge and tools that are now at our disposal, HIV is a completely preventable infection.
Identified in 1983, HIV belongs to a virus family known as 'retroviruses', which use the enzyme reverse transcriptase to turn viral RNA into DNA which can then be merged into the genetic material of the host cell. HIV exists in two types – HIV-1 and HIV-2 – with HIV-1 being responsible for the majority of HIV infections worldwide. HIV-2 infection is found largely in West Africa with some spread to Europe, particularly France and Portugal. Both HIVs result from a number of different cross-species transmissions of simian immunodeficiency viruses (SIVs) that infect African primates. Unless otherwise noted, the term 'HIV' refers to HIV-1.
HIV-1 is classified into four distinct subtypes – M, N, O and P – each reflecting a specific cross-species transmission. The transmission event that involved SIV crossing from chimpanzees to human beings in south-eastern Cameroon is thought to have given rise to HIV-1 group M, which is the major subtype accounting for 98% of global infections and the principal cause of the AIDS pandemic. Within subtype M, there is further viral diversity with different 'clades', denoted A–K, which have geographical differences in origin and distribution.2
HIV-1 and 2 are both transmitted horizontally through direct contact with body fluids, including blood, semen and vaginal fluids, or vertically from a mother who has HIV-1 to her child during pregnancy, delivery or through breast milk. The majority of HIV infections are transmitted sexually via semen, cervical secretions and blood.
Once acquired, the human body cannot get rid of HIV and no effective HIV cure exists, which means that HIV is present for life. However, by consistently taking anti-HIV medicine (called 'antiretroviral therapy' or 'ART'), people with HIV can live long and healthy lives and become non-infectious, preventing transmission of HIV to their sexual partners and children.
The human immunodeficiency virus (HIV) evolves rapidly owing to the combined activity of error-prone reverse transcriptase, recombination and short generation times, leading to extensive viral diversity both within and between hosts. This diversity is a major contributing factor in the failure of the immune system to eradicate the virus, and has important implications for the development of suitable drugs and vaccines to combat infection.3 This also has obvious biological implications as untreated patients eventually lose control over the virus and progress to AIDS. The rate of progression to AIDS is determined by the level of HIV replication and host factors (see section on Natural History on p.5). Some HIV mutations that develop while a person is taking HIV medicines can lead to drug-resistant strains of HIV. Once drug resistance develops, medicines that previously controlled a person's HIV are no longer as effective. In other words, the HIV medicines cannot prevent the drug-resistant HIV from multiplying. Drug resistance can cause HIV treatment to fail. Drug-resistant HIV can be transmitted from person to person or develop after a person starts taking HIV medicines (see Point 14.11b).
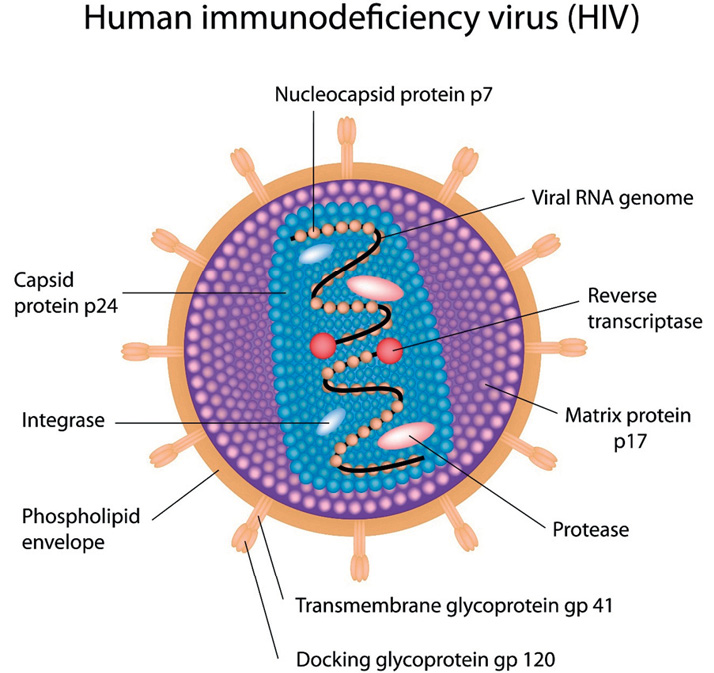
Figure 1a: The structure of HIV
Source: Dreamstime, Structure of HIV, accessed 28 January 2020, available at <https://www.dreamstime.com/stock-photography-structure-hiv-image23617032>.
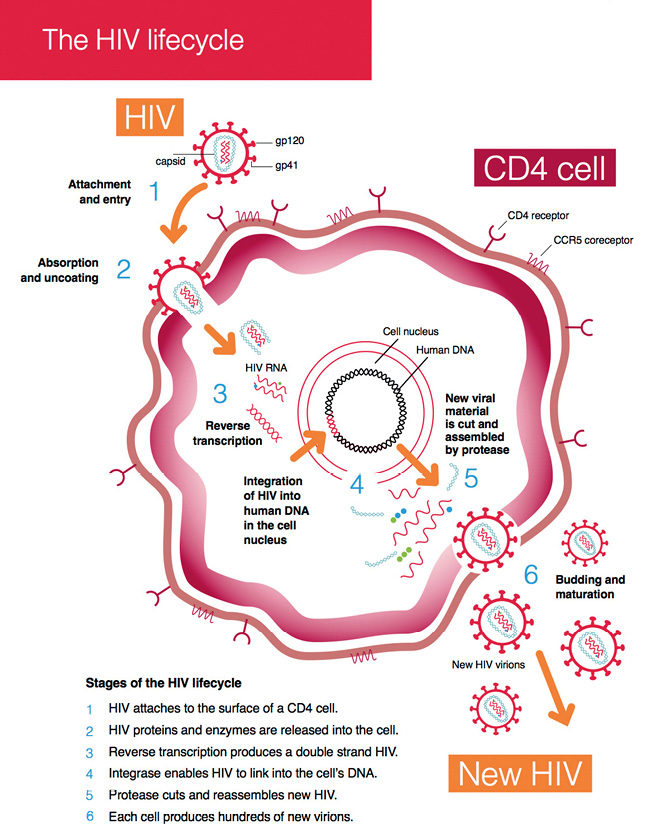
Figure 1b: The HIV lifecycle
Source: Collins, S 2019, The HIV Lifecycle, accessed 28 January 2020, available at <http://i-base.info/guides/wp-content/uploads/2017/06/ART-in-pictures-2-HIV-lifecycle.png>.
AIDS stands for 'Acquired Immune Deficiency Syndrome' and is a set of illnesses that occur at the late stage of HIV infection. It is diagnosed when the body's immune system is so severely damaged by HIV that serious infections can no longer be controlled or resisted. AIDS and HIV are not the same – AIDS is the clinical condition of which HIV is the causative agent.
A person with HIV is considered to have AIDS when:
More detail can be found on the stages and classification of HIV in Points 14.6, 14.9 and 14.13.
Today in the UK, most people with HIV do not develop AIDS because they start taking effective medication that treats HIV, anti-retroviral therapy (ART), before the immune system is badly damaged. Effective daily ART stops the activity and progression of HIV. Without ART, people with AIDS typically live for about three years. Once someone has an opportunistic illness, life expectancy without treatment falls to about one year. ART can still help people at this stage of HIV infection, and can be life-saving. However, people who start ART soon after they acquire HIV experience more benefit – thus the importance of HIV testing, early diagnosis and rapid initiation of therapy.
The natural history of untreated HIV infection is divided into early, chronic and advanced stages which are associated with progressively worsening immunodeficiency:
.png)
Figure 2: The natural course of HIV infection
Source: Wikimedia Commons, HIV-Timecourse, accessed 28 January 2020, available at <https://commons.wikimedia.org/wiki/File:HIV-timecourse_simple.svg>.
Standardised case definitions of HIV infection have been established by The World Health Organization (WHO)10 and by the US Centers for Disease Control and Prevention (CDC) classification.11,12 Their key application is public health monitoring and surveillance rather than clinical diagnostic tools. The most frequently used system is Centers for Disease Control (CDC) 1993 classification. Although the CDC classification was updated in 2014 the 1993 CDC classification remains is the most frequently used and is described below. The most recent (2014) CDC classification has 5 stages (Stages 0 – 4 or unknown), includes a combination of HIV positive laboratory results, the CD4 count / percentage, and the presence of clinical stage 3 defining conditions.13
Symptomatic HIV infection includes conditions listed in either categories B or C, however only the conditions in category C are considered AIDS defining.
In the UK the definition of AIDS requires the presence of an AIDS defining clinical condition. (category C list). A CD4 count below 200 alone is not AIDS defining.
In the USA a CD4 count below 200 is AIDS defining whether or not a category C condition is present.
Table 1: Summary of Centers for Disease Control and Prevention's '1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults'11
|
Absolute CD4 count (/mm3) |
A |
B |
C |
|
Asymptomatic or persistent generalised lymphadenopathy or acute, symptomatic (primary) HIV infection |
HIV-related conditions that are not A or C |
Clinical conditions listed in AIDS surveillance case definition |
|
|
>500 |
A1 |
B1 |
C1 |
|
200–499 |
A2 |
B2 |
C2 |
|
<200 |
A3 |
B3 |
C3 |
|
CLINICAL CATEGORY A |
CLINICAL CATEGORY B |
CLINICAL CATEGORY C AIDSDEFINING DISEASES |
|
Asymptomatic HIV infection Acute, symptomatic (primary) HIV infection Persistent generalised lymphadenopathy (PGL) |
Symptoms or signs of diseases that do not fall into Category C but are associated with a disturbed cellular immunity. Among these are:
|
AIDS defining diseases:
|
|
|
How HIV and AIDS are diagnosed, and how this has changed over the years. Included are descriptions of the tests and procedures used to effect diagnoses, and an analysis of how reliable the various diagnostic tests have been over the years.
The majority of HIV, hepatitis B and hepatitis C infections are initially diagnosed using enzyme immunoassays (EIAs). These techniques are widely used for screening large groups of people, as they are relatively inexpensive and can be highly automated. These assays are also known as 'serological assays' as they are usually performed on blood serum or plasma samples, but may also be performed on capillary/venous whole blood and oral fluid. Other serological assay formats include rapid diagnostic tests (RDTs), chemiluminescence immunoassays and electro-chemiluminescence immunoassays. The most recent assay format is the Chemiluminescent Microparticle Immunoassay (CMIA). Most serological assays are designed to detect specific antibodies which are produced in response to infection but, in the case of hepatitis and HIV, assays have also been designed to look directly at protein components (HBsAg, HCVcoreAg, p24 (HIV)) of the virus, or a combination of both antigen and antibody (for example, fourth generation HIV assays). The principle of any EIA is that an antigen or antibody which will specifically bind to the complementary antigen or antibody of interest (for example, hepatitis B surface antigen [HBSAg] or hepatitis C antibody) is immobilised on a solid surface – for example, a 96-well plate or a bead. If the antigen or antibody of interest is present in the serum, it will specifically bind to the immobilised complementary antigen/antibody. This can then be detected by using a secondary antibody which specifically binds to the antigen or antibody of interest and which has an enzyme attached to it. A chemical is added to be converted by the enzyme into a colour or a fluorescent or electrochemical signal. The amount of signal produced can be quantified and gives a measure of the amount of antigen or antibody in the blood sample. Between each step, the plate is washed with a mild detergent to remove any antigens or antibodies that are non-specifically bound. This is known as a 'sandwich ELISA' or 'indirect ELISA' (see Figure 3 below).
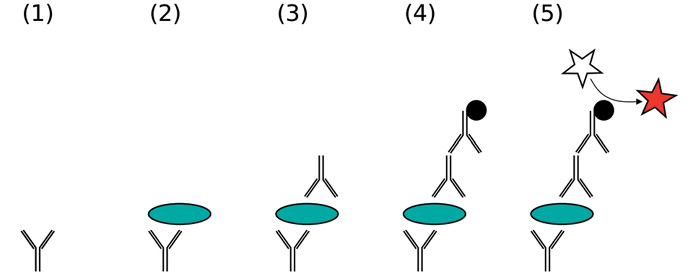
Figure 3: A sandwich ELISA.
Notes:
1. Vinocur JM, 2006 A Sandwich ELISA, accessed 28 January 2020, available at <https://en.wikipedia.org/wiki/ELISA#/media/File:ELISA-sandwich.svg>.
2. (1) Plate is coated with a capture antibody; (2) sample is added, and any antigen present binds to capture antibody; (3) detecting antibody is added, and binds to antigen; (4) enzyme-linked secondary antibody is added, and binds to detecting antibody; (5) substrate is added, and is converted by enzyme to detectable form.
The other key group of diagnostic assays for HBV, HCV and HIV are molecular assays, or nucleic acid testing (NAT) – for example, polymerase chain reaction (PCR) or nucleic acid sequence-based amplification that can detect very small quantities of viral nucleic acid (RNA or DNA). As well as detecting the presence of the virus, these assays can provide quantification of the virus in either virus copies/ml or, more recently, in the interest of standardising assays as international units IU/ml). These assays detect DNA or RNA by targeting a specific segment of the virus, which is then amplified. The amplification step enables the detection of low levels of the virus in the original specimen, which might not otherwise have been detectable. Laboratory-based technologies for NAT require sophisticated equipment, rigorous laboratory conditions and specimen collection, and highly trained staff who can perform precision steps and avoid contamination. NAT technologies are typically used to detect the presence of the virus, and to determine whether the infection is active and the individual would benefit from antiviral treatment. NAT technologies are also used to determine when antiviral treatment should be discontinued (due to non-response or resistance) or to confirm virological cure (HCV) or effective suppression (HBV). These assays are high cost compared with serological assays. Specialist virology laboratories were providing non-commercialised NAT-based assays (qualitative) from the early 1990s, often developed 'in-house', with more automated and quantitative assays becoming available in the second half of that decade. More sensitive and highly automated assays using real-time PCR technology have been used routinely since the mid to late 2000s.
Key attributes of any diagnostic test are the sensitivity (that is, the extent to which a test correctly identifies those with the disease [true positive rate]) and specificity (that is, the ability of the test to identify those without the disease [true negative rate]). A test with 100% sensitivity correctly identifies all those with the condition of interest; anything less than a sensitivity of 100% will mean that an individual may go undetected (false negative). A test with 100% specificity correctly identifies all those without the condition of interest; anything less than 100% will mean that an individual will be incorrectly diagnosed as being test positive (false positive). In general, screening tests designed for the diagnosis of HBV, HCV and HIV have a high sensitivity so that individuals with the diagnosis are not missed, which is important when such tests are used in the setting of blood donor screening. However, sensitivity and specificity of a test only describe how well the test performs against the gold-standard test for that disease. In order to assess how well a test performs in a particular population, where the prevalence of the disease may vary, it is necessary to understand the positive and negative predictive value of the test. The positive predictive value (PPV) of a test is the probability that, when a person's test result is positive, they truly have the infection/disease, whereas the negative predictive value (NPV) describes the probability that, when a person's test result is negative, they truly do not have the infection/disease. Generally, a higher prevalence of the disease in the population will increase the PPV and decrease the NPV. When a test is applied to a population with a low prevalence of the disease in question, unless the test has 100% specificity, the number of false positive results will be higher than when testing a population with a high prevalence. It is therefore important to design screening tests with a high sensitivity, and to perform further confirmatory tests with a high specificity before confirming the diagnosis. Before a confirmed diagnosis is made, further confirmatory tests with a high specificity are required. As the sensitivity of EIAs has increased, the window period (the time from infection with the virus to the appearance of measurable virus antigen or antibody) has decreased.
Clinical cases of HIV infection and AIDS were first recognised in 1981. The recognition of cases in intravenous drug users, haemophiliacs and infants born to mothers with AIDS suggested a blood-borne as well as a sexually transmitted pathogen. In May 1983, Dr Luc Montagnier of the Pasteur Institute in Paris isolated a virus which he named 'LAV' (lymphadenopathy-associated virus) that he believed to be the causative agent of AIDS. His work, although not fully appreciated at the time, became the basis for the development of tests to detect the virus and its antibodies.
In April 1984, Dr Robert Gallo of the National Institute of Health in the USA and his team of researchers isolated the virus that was the causative agent of AIDS. He named this virus 'HTLV-III' (human T-cell lymphotropic virus). This was the same virus that Dr Montagnier had found and, while at the time it was announced that the isolation had occurred independently of Dr Montagnier's work, it was later found that Dr Gallo's discovery was based, in part, on a sample of the virus provided by Dr Montagnier's laboratory. Dr Gallo produced a reagent capable of reacting with HTLV-III antibody present in blood serum. He was therefore able to test blood samples for the presence of HTLV-III antibody. A few weeks later, a third scientist, Dr Jay A. Levy of the University of California at Berkeley, also isolated the virus, which he named 'ARV' (AIDS-related virus). Eventually, in 1986, the virus became known as 'HIV' (human immunodeficiency virus), and for convenience that name is used throughout this chapter. Soon after Dr Gallo developed the test for HIV antibody, he shared his expertise and resources with the Canadian government, which developed a similar test at the Laboratory Centre for Disease Control in Ottawa in the summer of 1984. This test was performed on only a limited basis in research laboratories. It was not widely accessible. HIV antibody test kits were licensed and available in the United States at the beginning of March 1985.
In 1984, two methods were commonly used to test blood samples for HIV antibody. The first was an enzyme-linked immunosorbent assay (EIA). No commercial kits were available. Each test had to be made by hand in the laboratory ('in-house' assays). The EIA test was prepared by taking the reagent, which contained the antigen (the substance capable of inducing an immune response) obtained from inactivated HIV, and coating it on a plate with a number of wells. Although inactivated and non-infectious, the reagent was still able to bind HIV antibodies. The test was performed by diluting serum from a person's blood sample and adding it to one of the wells on the plate. If the person's blood contained HIV antibody, the serum would react with the HIV antigen on the plate. The reaction was made visible by adding reagents that caused a colour change.
The second, or confirmatory, test for identifying HIV antibody available in 1984 was the Western blot test. This is a much more complicated test, more difficult and more expensive. It is performed by separating, using an electric field, 'disrupted' HIV into its various proteins, transferring the proteins to paper, and adding the sample being tested to see whether antibodies in the sample will react with any of the HIV proteins. The various proteins are illustrated on paper as a line each. The completed test resembles a bar code. The western blot test was not confirmatory in the strict sense of the word because, like the EIA test, it tested for HIV antibody rather than the virus itself. It was, however, much more specific because it showed each of the proteins to which a particular sample reacted. Although the Western blot test was widely used, the interpretation of its results did not become standardised until the latter part of the 1980s. The cost of performing a Western blot test was approximately $100. By comparison, an EIA test cost about $4.
The World Health Organization (WHO) recommends replacing Western blotting and line immunoassays with simpler tests in HIV testing services. These simpler tests include RDTs that can be used at the point of care, and EIAs.
These tests get results to the client faster, produce accurate results more often, cost less, can be performed by various cadres of health providers, and can thus facilitate greater access and uptake of HIV testing services among those who need it most.
The first test in an HIV testing strategy and algorithm should have the highest sensitivity, followed by a second and third test of the highest specificity.
There are three main stages following HIV infection in an untreated individual. These are characterised by clinical symptoms and biological markers that also offer the opportunity for use in diagnosis and monitoring using laboratory testing. HIV infection is usually diagnosed by demonstrating the presence of antibodies against HIV-1 or HIV-2 with AIDS being primarily a clinical diagnosis. The clinical manifestation of infection with HIV-1 is the progressive loss of CD-4 positive lymphocytes. The resulting defect in cellular immunity leads to the development of opportunistic infections and malignancies that characterise AIDS.
This phase is characterised by rapid multiplication and spread of the virus in the body, which may take about 2 to 4 weeks following infection. During this stage, there is a burst of viral replication, with shedding and peaking of p24 antigen (Ag) in blood. HIV RNA is also detectable at this stage. During this phase, some people experience flu-like symptoms, such as headache, fever, and rash. The period from infection to the appearance of HIV-Ab (seroconversion) is known as the 'window period'.
This phase is characterised by continued viral multiplication at low levels, and the person with HIV may not experience any clinical symptoms. The host immune system produces HIV antibodies, which coincides with a decline in the HIV RNA viral load to a steady state. Free p24 antigen levels also fall, as p24 is bound by antibodies to form an antibody-p24 antigen complex. If a patient remains untreated, as viral replication continues, CD4 cells, which serve as host target cells for viral replication, are gradually destroyed, leading to a decline of CD4 cell numbers and the development of symptoms.
This phase is characterised by continual viral replication and depletion of CD4 cells, leading to a weakened host immune system, and is also characterised by opportunistic infections and other clinical symptoms.
The biological markers HIV RNA, p24 antigen, HIV antibodies and CD4 cells are used in laboratory diagnostics for HIV for various applications, including:
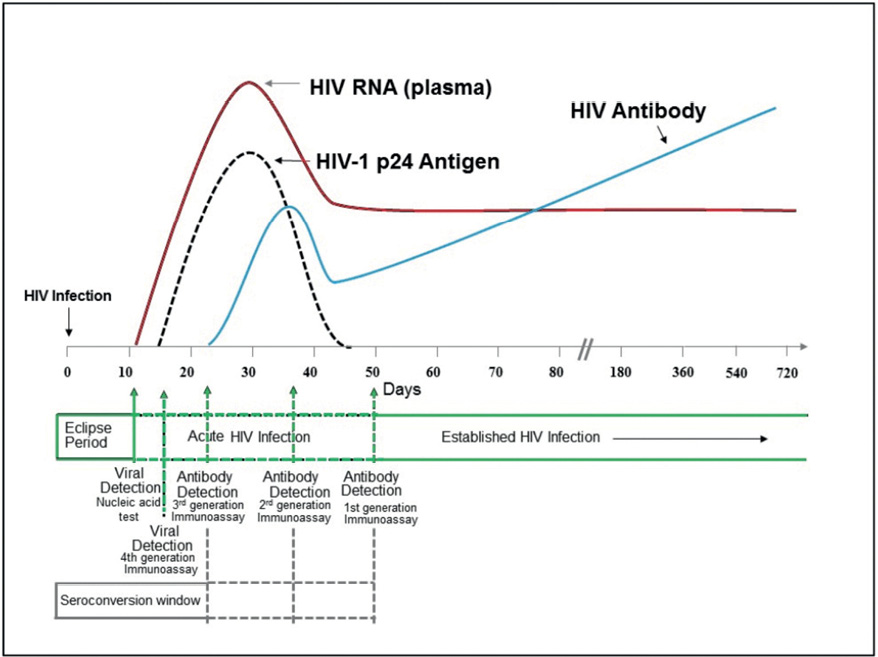
Figure 4: Sequence of appearance of laboratory markers for HIV-1 infection
Note: Units for vertical axis are not noted because their magnitude differs for RNA, p24 antigen, and antibody. Modified from MP Busch, GA Satten (1991) with updated data from Fiebig (2003), Owen (2008), and Masciotra (2011, 2013).
Source: Centers for Disease Control and Prevention 2014, Laboratory testing for the diagnosis of HIV infection: updated recommendations, accessed 28 January 2020, available at: <https://stacks.cdc.gov/view/cdc/23447>.
HIV infection is usually diagnosed by demonstrating the presence of antibodies against HIV-1 or HIV-2.
Since the mid-1980s, there have been five generations of EIAs using different antigen preparations and detection chemistries to provide accurate screening for blood banks and centralised laboratories with a high specimen volume (Figure 5).
The first-generation assays were designed in around 1984 and used antigens derived from whole viral lysates from HIV positive cultures for the detection of IgG antibodies. These assays had a high sensitivity but, due to the presence of impurities in the crude antigen lysate preparations, specificity was as low as 10% (that is, only 1 in 10 positive results were truly positive). Later confirmatory tests with high specificity, such as immunofluorescence assays or Western blotting, were introduced to eliminate false positivity.
In the second-generation assays, synthetic peptides or recombinant proteins derived from the immunodominant regions (IDR) of HIV-1 proteins and the gp36 protein of HIV-2 were used to increase sensitivity and reduce false positivity.
The third-generation assays, such as the Genetic Systems HIV-1/HIV-2 Plus O EIA, used a sandwich format and a variety of antigens to capture HIV-1 and HIV-2 antibodies in serum. These antigens included recombinant p24, gp160 derived from HIV-1 group M, a recombinant peptide from HIV-2 gp36 IDR, and a synthetic peptide from HIV-1 group O. In addition to IgG, the third-generation assays also detected early HIV-1 IgM antibodies and further reduced the window period.
The fourth generation EIAs, such as the Abbott Architect HIV Ag/Ab Combo assay, use fully automated CMIA technology to simultaneously detect HIV-1 p24 antigen and antibodies to HIV-1 (groups M, N and O) and HIV-2. The detection of p24 antigen shortens the window period and increases the chances of early detection of HIV infection. Instruments such as the Abbott Architect provide random-access high-through-put capability so that specimens can be rapidly tested on arrival in the laboratory with results in 30 minutes.
Fifth-generation EIAs, such as the Bio-Rad BioPlex 2200 HIV Ag-Ab assay, use multiple sets of magnetic beads coated with p24 monoclonal antibodies and epitopes specific for HIV-1 (groups M, N and O) and HIV-2 (42), and allow identification of each individual component of the assay. This facilitates identification of people acutely infected with p24 antigen in the window period in a single test. Individuals with HIV-1 or HIV-2 can also be identified for quick confirmation and linked to HIV-1- or HIV-2-specific ART.
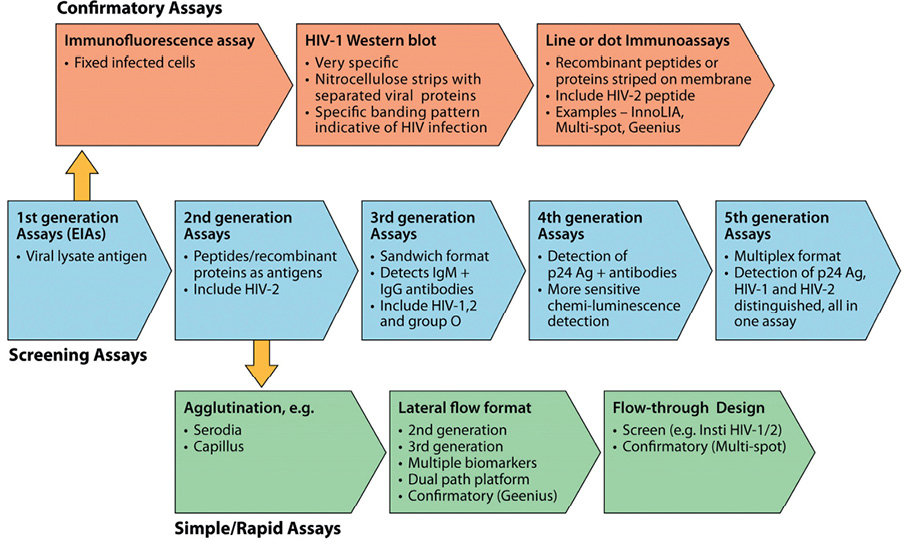
Figure 5: Generations of EIAs for primary HIV infection diagnosis.
Note: Historical evolution of serolgic assays for HIV diagnosis. Shown are five generations of screening assays using an EIA format for high-throughput processing. Supplemental assays for confirmation of infection used immunofluorescence, WB, and, more recently, simple line or dot immunoassays. Rapid assays for POC testing were initially agglutination tests and later of a lateral flow format and flowthrough design.
Source: Parekh, BS, Ou CY, Fonjungo PN et al. 2018, 'Diagnosis of Human Immunodeficiency Virus Infection', Clinical Microbiology Reviews 32 (1) e00064-18, DOI: 10.1128/CMR.00064-18.
In 2017 8% of those living with HIV in the UK were unaware of their diagnosis, a fall from 24% in 2013. 43% of people newly diagnosed in the UK in 2017 were diagnosed late that is, they had a CD4 count below <350 cells/mm3 and in 33%, the CD4 count was <200 cells/mm3, putting them at high risk of HIV-associated pathology.85
Given the benefits of treatment of HIV, both to an individual's health and to the wider public health, best outcomes depend upon ensuring that all those with HIV are diagnosed promptly and can rapidly access treatment and care. People who test HIV negative and who remain at risk should be able to access appropriate combination prevention interventions, including pre-exposure prophylaxis [PrEP]) that will allow them to avoid future acquisition. For those at particular risk repeat testing is critically important in ensuring early diagnosis and rapid initiation of therapy. Current approaches aim to expand and normalise HIV testing and avoid exceptionilsisng the condition. Ongoing barriers to testing include lack of knowledge / information, HIV associated stigma and reluctance to offer testing by healthcare professionals.
UpToDate best practice approaches to HIV testing have been published by NICE and Public Health England.86 Latest testing guidance (at the time of writing in still consultation draft form) is available from the British HIV Association (BHIVA) the British Association for Sexual Health and HIV (BASHH) and the British Infection Association (BIA).87 In summary all healthcare workers should be able to offer an HIV test in their setting. 'Opt-out' testing, whereby attendees are given information that they will be automatically tested unless they actively decline, aims to increase coverage and normalise HIV testing. Opt-out models of testing in acute care settings have been shown to be acceptable, feasible and, with appropriate resources, sustainable. Specific pre-test discussion is not required however GMC good practice for any medical intervention should be followed, i.e. individuals should be made aware that they will be tested for HIV and informed how they will receive their result; for clinical settings, opt-out testing is the most effective method to increase testing coverage.
Although a majority of new HIV diagnoses are made within sexual health and genitourinary medicine services an increasing proportion are made in primary care and other clinical and community settings. In 2016 where data are available (n=240,757), GUM clinics tested the greatest proportion of individuals for HIV (49.3%), with a further 18.9% tested in general practice, and 10.8% tested in other known hospital wards. The highest proportion of positive tests were among specialist HIV services, unspecified wards and specialist liver services (31.9%, 7.1% and 1.6% respectively).88
In all settings, irrespective of who is delivering the testing, there should be clear, agreed pathways to HIV treatment and care services delivering timely linkage to care. For those testing negative who remain at risk there should be clear pathways to prevention services.
HIV testing is recommended for:87
All sexual partners of an individual diagnosed with HIV should be offered and recommended an HIV test unless all episodes of sexual contact were known to be protected by Treatment as Prevention (i.e. the person living with HIV was on ART with a maintained undetectable viral load).
People with HIV should be provided options on how their partners can be contacted, as well as time to consider the best options, based on their needs (see also Point 14.17). People who do not want their partners to be contacted or need time to consider should be supported in their decision. Where feasible and acceptable to the client, provider-assisted referral should be prioritized, as it is highly effective and provides the opportunity to offer comprehensive prevention interventions to partners who are HIV-negative but remain vulnerable to HIV acquisition. Ensuring testing for all untested biological children of people who are HIV positive is required
All pregnant women in the UK are offered opt out HIV screening as part of their antenatal care. Uptake of HIV screening among women who attend for antenatal care is very high (>99%) and positivity remains low (0.013%).82
Donor selection criteria aim to ensure that the population eligible to donate blood are at low risk for viral infections which have potential to be transmitted via transfusion. Data confirm this to be an effective strategy, for example, the UK prevalence of HCV infection is 0.67% but the prevalence among first-time blood donors meeting donor selection criteria is 0.03%. Testing strategies employed to assess the suitability of donated blood for transfusion are therefore used in the context of very low chance of infection but, given that it is essential that all infections are detected, assays with high sensitivity are used.
Testing for HIV in donated blood was performed from the mid-1980s by screening for the presence of anti-HIV antibody. Advances in testing technologies led to the implementation of combined testing for anti-HIV antibody and HIV antigen in 2001, with NAT assays introduced in 2003. The UK Transfusion Guidelines89 suggest the use of these combination HIV Ab/Ag serological assays because their use allows detection of the p24 antigen in samples which may not yet contain anti-HIV antibodies, thereby shortening the residual window period and reducing the risk that an infected donation may be missed. The UK requirement for the minimum level of sensitivity for the performance of HIV 1+2 serological screening is that a positive result should be obtained with the UK anti-HIV 1 working standard, which can be obtained from the National Institute for Biological Standards and Control (NIBSC). This standard must be included in each series of tests as a positive control, as well as any manufacturer's controls. These steps ensure validity of test results. HIV Ab/Ag combination assays used by the blood services report sensitivity of 100% and specificity of >99.5%. Any samples from donations which are found to be reactive are then subject to confirmatory testing.
NAT assays are employed to identify the presence of HIV-RNA within a sample which may identify cases of early infection in which HIV Ag/Ab is not yet positive. There is no specific UK requirement for the minimum sensitivity of HIV NAT. However, a standard is available and all assays must be appropriately controlled. Samples from individual donations are pooled for initial testing. If a pool is reactive, the samples which make up the pool are tested individually to identify the reactive donation(s).
There is considerable variation in the symptoms and signs that a person may develop when they first acquire HIV – some people have no symptoms whilst others become extremely unwell.90
The early stage of infection is taken as the first six months following viral acquisition. During this time, HIV undergoes very active replication and the level of virus in the body (the viral load) is very high, which makes this a period of elevated infectiousness.
People who are seen rapidly after HIV acquisition may present before they have detectable levels of routine diagnostic HIV-specific antibodies, which can make the diagnosis difficult.
The level of viral replication stabilises and reaches a steady state known as 'the viral set point' by approximately six months of infection. The viral set point can vary considerably between individuals and is correlated with the rate of progression of HIV disease, with a higher viral set point being linked to more rapid progression.
The stages of the development of laboratory markers of HIV and changes in viral load concentrations during early HIV infection are classified by the Fiebig classification.91
The first two weeks immediately following infection are typically 'silent' without any symptoms or signs. Between two and four weeks following infection, a proportion of people will develop the symptoms and signs of 'acute' HIV infection (also known as 'primary HIV infection' or 'acute retroviral syndrome'), which was first described in 1985.92 Estimates of the proportion of people with early HIV infection who experience symptoms vary widely from fewer than 40% to more than 90%.93,94
The symptoms most frequently described are a raised temperature, a sore throat, mouth ulceration, enlarged lymph nodes, aching muscles and joints, and tiredness. A short-lived, faint pale pink rash is sometimes seen. Nausea, diarrhoea and weight loss can occur. Neurological symptoms are common and may include headache and aversion to light (photophobia). In rare cases there may be signs of meningitis or of direct brain infection (encephalopathy). In most people, the illness lasts up to three weeks, resolves on its own and recovery is usually complete.
In some people, the syndrome may last longer or be more serious. Acute HIV infection can occasionally lead to profound immunosuppression due to the marked fall in the CD4 cell count, which in turn may make people susceptible to unusual or opportunistic infections. Prolonged or more serious acute infection has been associated with poorer long-term prognosis.
Acute HIV infection is frequently undiagnosed or misdiagnosed as the non-specific nature of many of the symptoms and signs means that it may be confused with other viral infections such as Epstein Barr virus (glandular fever). Because symptoms are unspecific and variable, the diagnosis of HIV infection is rarely made without additional testing. Possible risks for HIV infection may not be routinely explored and symptoms may occur when antibodies to HIV may not yet have been produced. Laboratory tests to identify other viral components such as p24 antigen or HIV RNA may be positive before a sensitive HIV antibody test. If early or acute HIV infection is suspected but standard diagnostic tests are negative, then repeat testing seven days later is recommended.
This section describes the period that may elapse between first acquiring HIV and symptoms of AIDS first emerging (a 'latency period') and what is known about any factors which may affect the length of this latency period either by shortening or prolonging it.
There is variation in the rate at which untreated HIV progresses. Following the early stage of infection, most people with HIV infection are asymptomatic for some years. This is known as 'clinical latency'. However, despite the absence of symptoms, the virus continues to actively replicate which results in ongoing CD4 cell destruction and progressive immune dysfunction. Without treatment, most people with HIV have a gradual decline in CD4 count over a period of approximately 8 to 10 years before the development of symptomatic disease, a CD4 count less than 200 cells/mm3 or to AIDS. Long-term HIV infection is associated with increased levels of inflammation and immune activation which contribute to both loss of CD4 cells and to elevated rates of number of non-communicable conditions amongst people with HIV.95,96
A subgroup of people with otherwise asymptomatic infection may have persistently enlarged lymph nodes (persistent generalised lymphadenopathy, PGL). The nodes are usually symmetrical, firm and non-tender. The spleen may also be enlarged. Similar disease progression has been noted in those with or without PGL. Nodes may disappear with disease progression.
In the absence of antiretroviral therapy, the factors that influence the period of clinical latency may be either pathogen or host related.
Viral factors: a higher viral set point and high baseline viral load are both associated with a faster rate of loss of CD4 cells and more rapid progression to symptomatic disease. The type and strain of HIV may be important, with evidence that the CD4 decline in HIV-2 is slower than in HIV 1 and the symptomatic period is longer. Differences in HIV 1 subtype have been implicated although the major geographical and social variables associated with differences in subtype complicate the picture. The type of cellular receptor that the virus uses to enter cells may be important with viruses that use the CXCR4 receptor leading to more rapid progression.
Host factors: CD8 lymphocytes are important in viral control – higher numbers and proportions of circulating CD8 lymphocytes are associated with reduced viral turnover and slower progression. People with cells that express the HLA -B57 marker have lower viral set points and slower disease progression. Older age has been associated with more rapid progression. Gender and pregnancy per se do not appear to have a direct impact on the rate of HIV progression. High levels of alcohol consumption have been inked to CD4 count decline.
A small number of people with HIV known as 'long-term non-progressors' may continue with a CD4 count within the normal range over many years. Within this group, a sub-population of people ('elite controllers') maintain a viral load that is less than 2,000 copies/mL or even to undetectable levels without therapy. Elite suppression occurs in approximately 1 in 500 people with HIV. This is not permanent and over time elite controllers lose virologic and immune control with consequent disease progression.
The mechanisms of elite control remain incompletely understood. Potential factors include infection with types of HIV that are less able to replicate, the possibility that individuals may have innate resistance to HIV-1 infection, as well as genetic variations that can modify various aspects of the host immune response.97
The presence of other infections such as tuberculosis, syphilis and parasitic worms has been associated with more rapid CD4 cell decline.
Hepatitis G (GBV-C) is caused by a small RNA virus with similarities to Hepatitis C Virus (HCV). There have been several reports associating GBV-C with a better outcome of HIV infection. In general, GBV-C/HIV co-infection is associated with lower HIV viral load, slower progression to AIDS and improved response to HIV treatment. These effects are more likely to be seen with advanced HIV infection (low CD4 counts).98 Theoretically, in HIV/HCV/GBV-C triple infected individuals, treatment of HCV with interferon could also lead to clearance of GBV-C which may, paradoxically, worsen outcome of HIV infection. However, this was not supported by clinical studies.
The natural course of HIV – in the absence of antiretroviral therapy – is shown in Figure 2. In an era when effective treatment for HIV is consistently available, where people with HIV are diagnosed promptly and are able to access lifelong treatment, HIV has evolved from a universally fatal infection to a long-term manageable condition with a life expectancy that can match that of the general population. In these settings, the complications of HIV associated with immunosuppression have become much less common whilst other conditions, notably coinfections and conditions associated with low-grade inflammation and with ageing, are seen more frequently.99
This response takes the natural history and complications of untreated HIV as its focus.
The spectrum of disorders associated with HIV infection is broad and the result of HIV-associated immune dysfunction, direct HIV effects and the drugs used to treat the condition, as well as coexisting conditions, including depression and anxiety and co-infections such as hepatitis. Health-related quality of life is lower amongst people living with HIV than the general population.
The level of HIV RNA, which reaches extremely high values shortly after primary infection, usually decreases to less than 1% of the maximum value at the time of first HIV antibodies and remains relatively stable for a number of years. This level is called 'the viral set point'. The level of the viral set point determines the speed of disease progression. The higher the viral set point, the faster the decrease of CD4 T cells.
CD4 T cells usually drop considerably during acute primary infection. Subsequent CD4 counts recover after a few months to values within the normal range, though pre-infection values are rarely reached. During the progressive course of HIV infection, a gradual decrease of CD4 T cells is observed.
Without treatment, over time as the viral load rises and the CD4 count falls, the immune system becomes increasingly dysfunctional and an array of problems can develop. These can include non-specific symptoms such as fatigue, weight loss and sweats, particularly at night, and conditions classified according to the CDC as Category B may develop (Table 1, please see also Figure 2.5 in the Krever report, Chapter 2).164 Among these, oral thrush, oral hairy leukoplakia and herpes zoster are particularly noteworthy, and HIV infection as an underlying diagnosis should always be taken into account. Conditions in Category B are not AIDS-defining. However, their occurrence is defined as symptomatic of HIV infection and suggests a disturbed cellular immune system.
With further immunosuppression, the person is susceptible to an increasing range of opportunistic infections and tumours, which meet the criteria for the diagnosis of AIDS (see CDC Category C classification list in Table 1).
While most patients with less than 1,000 HIV RNA copies/ml are usually not affected by AIDS even 12 years after primary infection, more than 80% of patients have developed AIDS only two years after infection if the viral load remains at levels above 100,000 copies/ml.100 The risk for AIDS-defining illnesses increases with time when CD4 T cells decrease below 200. In the pre-ART era, the average time between the first manifestations of AIDS and death was two to four years.
Apart from the level of HIV RNA and CD4 T cell count, the age of the patient is another important risk factor for progression to AIDS. The increasing risk with age has been described to be similar in haemophiliacs and homosexual men.101 Regarding AIDS-defining events, some differences exist between different affected key populations. For example, Kaposi sarcoma is rarely found in haemophiliacs and mostly found in homosexual men. Table 1, Categories B and C, lists a number of health complications.
There are, however, a number of health complications listed which can also be observed independent of HIV. The question mostly is whether the medical condition occurred prior to or after HIV infection had taken place. For example, epilepsy can develop independent of HIV but can also be a sequelae of cerebral toxoplasmosis which is an AIDS-defining event.
With early intervention of effective ART, the majority of people with HIV in resource-rich settings begin treatment whilst asymptomatic, before the onset of significant immunosuppression. Progression to an AIDS-defining event is now uncommon.
In an individual person, the consequences of HIV-related immune dysfunction depend on at least three key factors:
HIV can also directly infect cells of the nervous system, skin, gut and kidneys which if untreated can cause direct organ damage. Examples include AIDS Dementia Complex, HIV-associated nephropathy (HIVAN) and dry, itchy skin.
HIV is related to abnormalities in the inflammatory response which are implicated in higher rates of allergic reactions and some autoimmune conditions (for example, psoriasis). HIV-associated inflammatory responses are also linked to the development of neurological, renal, cardiovascular and bone disorders which are more prevalent amongst people living with HIV. Even with effective antiretroviral treatment, people with HIV have higher levels of multimorbidity occurring at younger age than those who are HIV negative. Frailty and its associated disabilities appear to occur at a younger age in people with HIV. Leading causes of hospital admission of people with HIV in Europe in 2017 included respiratory illness, psychiatric conditions, and cardiovascular, renal and neurological disorders. Data from the UK show 75% of those living with HIV have at least one other long-term condition including mental health conditions, hypertension, lipid disorders and diabetes. Identification of comorbidities, their risk factors and interventions for prevention through an integrated, outcomes-focused person-centred approach is central to HIV care (see Point 14.13).
People who start ART when profoundly immunosuppressed may experience inflammatory symptoms associated with the recovery of their immune system, known as 'immune reconstitution inflammatory syndrome (IRIS)'. Symptoms of existing infections may worsen, or symptoms of undiagnosed conditions may emerge (see also Point 14.12).
Ensuring that the diagnosis of HIV is made promptly is very important in securing best outcomes. Recognising the conditions, symptoms and signs that are associated with HIV in people who are not yet diagnosed, and recommending HIV testing is critically important in reducing rates of late and undiagnosed HIV. Anyone presenting to any clinical service with any of the stage 3/AIDS-defining conditions should be immediately tested for HIV. Other conditions in which the prevalence of undiagnosed HIV is more than 0.1%, and where an HIV test is indicated, include sexually transmitted infections, malignant lymphoma, herpes zoster, hepatitis B or C (acute or chronic), unexplained lymphadenopathy, mononucleosis-like illness, community-acquired pneumonia, unexplained leukocytopenia/thrombocytopenia lasting more than four weeks and seborrheic dermatitis/exanthema.102
Lymphocytes are white blood cells of which T lymphocytes are one type. Types of lymphocytes can be distinguished by their molecular markers which are known as 'cluster determinants'. T lymphocytes that 'help' other parts of the immune system carry cluster determinant 4 and are known as CD4+ T helper lymphocytes. It is these cells that are both targeted by and gradually become depleted by HIV over time. These cells are responsible for the co-ordination of the immune response to infection and for immunological memory. Both the absolute CD4 count and its percentage of total lymphocytes fall as HIV progresses. In a person with a healthy immune system, the CD4 count range in blood is between 500 and 1,500 cells/mm3. As the immune system becomes progressively more damaged by HIV infection, the CD4 count falls and the risk of complications of HIV increases, with the greatest risks occurring at counts below 200 cells/mm3. Up until 2015, CD4 counts played an important part in decision making for when to start antiretroviral therapy – today, however, patients are recommended to start ART irrespective of CD4 count. The CD4 count remains important for assessing stage of disease and determining interventions such as prophylaxis against opportunistic infections and malignancy and the safety of live vaccines.
Monitoring in the UK follows the British HIV Association Guidance.103 The baseline CD4+ T cell count and percentage should be measured at first presentation for clinical staging and to assess the absolute urgency to start ART. As per current treatment guidelines, most patients now start treatment within a few weeks of HIV diagnosis, many within a few days. For those presenting with more advanced disease (as determined by CD4+ count) or certain AIDS-defining conditions, the urgency to start treatment is higher. On treatment, the CD4 count is monitored every three to six months for those patients with advanced disease. However, once virologically stable on ART and with a CD4 consistently above 350cell/mm3, routine monitoring of CD4 counts is no longer recommended.
The significance of the CD4 count for the person with HIV is as a marker of the level of immunosuppression, the relative risk of complications developing and, until recently, a guide to when to start and/or the urgency of starting ART. It is also useful for making decisions on other preventative interventions including vaccinations.
The amount of HIV RNA measured in the circulation of a person with HIV is known as 'the HIV viral load'. The viral load is highest in primary HIV infection but by about six months after acquisition it has stabilised to a 'set point'. Higher viral loads are linked to more rapid loss of CD4 cells and are co-related with infectiousness. The viral load level immediately prior to starting ART influences the choice of agents used in ART, in that several treatments have reduced efficacy at a viral load >100,000 copies/ml and are best avoided if the viral load is higher.104 The viral load is the standard marker of antiretroviral treatment efficacy. Effective antiretroviral therapy suppresses viral activity and the viral load falls to a level where assays cannot detect RNA, usually four to six months after starting ART. This is referred to as an undetectable viral load and the aim of ART is securing this level of viral suppression for the long term. Although there is generally a good correlation in the measurements between different manufacturers' assays, their lower limit of quantitation differs (range 20–75 copies/ml). A viral load below 50 copies/ml is generally considered to be 'undetectable'.
BHIVA guidelines103 recommend that viral load is measured at time of diagnosis and then at intervals of four to six months if ART is not started. Following the initiation of ART or changes in therapy, a reduction in viral load should be seen by four weeks, falling to lower levels by 10–12 weeks, when repeat viral load testing should be carried out, before becoming undetectable at four to six months. Once undetectable and the patient is virologically stable on ART, viral load is routinely monitored every six months. People with HIV whose viral load is consistently undetectable on ART are not able to pass HIV to their sexual partners and vertical transmission is prevented.105,106
Even with excellent adherence, occasionally the viral load can transiently become detectable at very low levels only to revert to undetectable again. This is referred to as a 'blip'. However, a viral load that is consistently detectable and/or rising to above 200 copies/ml indicates virological failure. The patient should be assessed for adherence and may need to switch therapy.
The significance of the viral load for the person with HIV is as a marker of the level of activity of their HIV infection, a guide to the appropriate antiretroviral drug regimen, a marker of the effectiveness of their ART and an indicator of the need to switch treatment. Once the viral load is consistently undetectable, the person with HIV is no longer able to pass on HIV to other people (Undetectable = Untransmissible, U=U)107,108 which is of major significance for the lives and the social, sexual and reproductive choices for people living with HIV and their partners.
The natural course of HIV – in the absence of antiretroviral therapy – is shown in Figure 2. Shortly after infection, an acute/primary infection syndrome is observed in some patients. This syndrome is characterized mainly by lymphadenopathy, fever, maculopapular rash and myalgia and usually does not last longer than four weeks (see Point 14.6 for more information).
The symptoms are unspecific and variable so that the diagnosis of HIV infection is rarely made without additional testing. A period of several years follows when most patients are clinically asymptomatic.
Thereafter, symptoms or diseases may occur, classified according to the Centers for Disease Control and Prevention (CDC) as Category B (Table 1; please see also Figure 2.5 in the Krever report, Chapter 2).164 Among these, oral thrush, oral hairy leukoplakia and herpes zoster are particularly noteworthy, and HIV infection as an underlying diagnosis should always be taken into account. Diseases of category B are not AIDS defining. However, their occurrence is defined as symptomatic of HIV infection and suggests a disturbed cellular immune system.
Later in the course of HIV infection, AIDS-defining illnesses occur at a median of 8–10 years after infection. Without highly active antiretroviral therapy, these illnesses eventually lead to death after a variable period of time.
The level of HIV RNA, which reaches extremely high values shortly after primary infection, usually decreases to less than 1% of the maximum value at appearance of HIV antibodies and remains relatively stable for a number of years. This level is called 'the viral set point'. The level of the viral set point determines the speed of disease progression. While most patients with less than 1,000 HIV RNA copies/ml are usually not affected by AIDS even 12 years after primary infection, more than 80% of patients have developed AIDS only 2 years after infection if the viral load remains at levels above 100,000 copies/ml.100 The higher the viral set point, the faster the decrease of CD4 T cells. CD4 T cells usually drop considerably during acute primary infection. Subsequent CD4 counts recover after a few months to values within the normal range, though pre-infection values are rarely reached. During the progressive course of HIV infection, a gradual decrease of CD4 T cells is observed. The risk for AIDS-defining illnesses increases with time when CD4 T cells decrease below 200. In the pre-ART era, the average time between the first manifestations of AIDS and death was two to four years. Apart from the level of HIV RNA and CD4 T cell count, the age of the patient is another important risk factor for progression to AIDS. The increasing risk with age has been described to be similar in haemophiliacs and homosexual men.101 With regard to AIDS-defining events, some differences exist between different affected key populations. For example, Kaposi sarcoma are hardly found in haemophiliacs and mostly found in homosexual men. Of the listed health complications many can be found in Table 1 under Category B or C. There are, however, a number of health complications listed which can also be observed independent of HIV. Most significant is whether the medical condition occurred prior to or after HIV infection. For example, epilepsy can develop independent of HIV but also can be a sequela of cerebral toxoplasmosis which is an AIDS-defining event.
Similarly, kidney stones can occur independent of HIV but can also be a side effect of antiretrovirals such as atazanavir, darunavir or indinavir. Gallstones are not HIV related.
It is important to highlight that, due to the transmission pathways for people with inherited bleeding disorders, HIV infections mostly occurred in the 1970s and 1980s and, as such, many individuals were exposed to first generation antiretrovirals which were associated with significantly more toxicity than subsequent regimens which involved some of the more recent drug classes. Within the NRTI (first drug class available) drug class mitochondrial toxicity was prominent including risk for development of lipoatrophy, anemia, pancreatitis, diabetes, polyneuropathy, myopathy and hepatic steatosis. Among the protease inhibitor first generation drug class gastrointestinal toxicity, kidney stones (indinavir), diabetes (indinavir) and dyslipidemia were the most common adverse events. For NNRTI it was drug rashes, hepatotoxicity and CNS-toxicity.
The mental health of people living with HIV is less good than that of the general population. Depression and anxiety are amongst the major disorders reported. Poor mental health has been linked to HIV-associated stigma.
With effective ART, people with HIV are living longer and are developing the disorders associated with older age. These conditions, including cardiovascular disease, hypertension, renal disorders, bone pathology and neurocognitive problems are more common and occur at an earlier age in people with HIV than the general population. There is a clustering effect, leading to multiple conditions occurring together (multimorbidity). This has a direct impact on the quality of life of people living with HIV and can lead to complications associated with multiple medications, co-ordination of care and quality of life.109
The way that an individual experiences the effects of HIV will differ depending on their particular circumstances, the stage at which the infection is diagnosed, co-existing conditions and the treatment interventions that are being made. Demographic, cultural and social factors will all influence the impact that HIV may have on an individual and their response.
HIV-associated stigma may prevent people from accessing the care and support that could make a difference, may lead to discrimination and self-stigma which can undermine people's self-esteem and self-worth, and can have a negative impact on mental health.
Today in the UK, HIV disproportionately affects communities in which people may already be underserved and/or marginalised, and the intersectionality of characteristics including gender, ethnicity, migration status, sexual orientation and substance use with HIV infection frequently creates a particularly difficult environment. The focus of the Inquiry is on those people who have acquired HIV from infected blood and blood products. It is important to recognise that, although there are commonalities amongst people who experience HIV infection, there is also considerable variation regarding the significance of various social and psychological impacts.
Today national guidelines on HIV testing advocate expanded HIV testing across healthcare settings, with the aim of benefiting both individual and public health. In the past, before the availability of effective treatment and in an environment of stigma, misconception, fear and discrimination, extensive consideration with a trained healthcare professional was recommended prior to undertaking the HIV test: 'pre-test counselling'. The focus of pre-test counselling was to individualise the implication of receiving a HIV positive result where, prior to treatment, there were few benefits to knowing the result and many challenging consequences of such a diagnosis. When the test result was received, further support and counselling were given. For those with a negative result, the focus of the discussion was on reducing activity that could increase the risk of HIV acquisition. If the result was positive, the post-test counselling session was an opportunity to offer support, assist with adjustment and establish appropriate referrals for ongoing care and management. Today, lengthy pre-test counselling is no longer recommended prior to testing. Guidelines are provided by The British HIV Association (BHIVA) to enable any clinician to perform an HIV test within good clinical practice and to encourage 'normalisation' of HIV testing and rapid referral to specialist services.
There were many in the 1980s, when HIV testing first became available, who were part of cohorts considered at high risk of having acquired HIV and were already established patients within the heath service – particularly those with haemophilia who had received factor products and intravenous drug users. Blood was tested, sometimes on stored samples, and patient consent not obtained. The benefits offered by pre- and post-test counselling in such circumstances would have been absent.
For further details on HIV testing procedures, see Point 14.17. For details on diagnostic tests, see Point 14.4.
Today, people diagnosed HIV positive in the UK will be referred rapidly into specialised services with the option of immediate effective treatments, care and support. Patients, partners and family members should receive information and support as required (see Point 14.17). Further clinical assessment and investigations will determine appropriate treatment options for consideration, and ongoing physical and mental health monitoring will be established. Discussions with experienced healthcare workers will assist patients with decisions related to disclosure of their HIV diagnosis to family, friends, workplaces, prevention of transmission to sexual partners or mother-to-child transmission, contraceptive and conception advice.
With effective treatments, the impact of HIV infection today is different from the pre-ART era, with significantly fewer HIV-associated physical symptoms and illness. Increasing social awareness and protective public policy has helped to change attitudes and reduce discrimination towards those living with HIV. However, many people have experienced and continue to experience social and psychological challenges as well as dealing with stigma. Both external stigma and self-stigma remain a major complication of HIV.
All long-term conditions have psychological impacts, both on those with the condition and those who are close to them. Ill health affects an individual's emotional state and their self-identity and may require major adjustments – for example, to accommodate pain, incapacity, long-term drug treatment and side effects, clinical interventions and changes to lifestyle. For some, such adjustments may be straightforward with personal and professional support; others may experience what is considered an adjustment disorder or stress response syndrome. Such a response can manifest as tearfulness, feelings of hopelessness, loss of motivation, and interest in work or normal life activities. Professional support at HIV diagnosis and consistently during ongoing care and management is required to prevent or offer continued support.
The extent of social disruption and hardship experienced by those with HIV infection is influenced by an individual's social circumstances, age, life stage, economic situation and social support. Today, with a life expectancy near to the same as the general population, HIV for many people has a different level of social psychological impact from that prior to effective treatment in the 1980s and 1990s when life expectancy was uncertain and prognosis poor. For younger people, school and educational attainment and achievement may have been negatively affected. HIV-related health issues had a direct impact on employment and career opportunities, because of absence from work due to ill health or due to stigmatising workplaces reducing employment opportunities. Challenge to employment resulted in financial hardship often borne by partners. HIV had an impact on travel due to ill health or because of visa restrictions. Property was unattainable because mortgages and health insurance were restricted. Friends, family members and social groups may have failed to offer the support expected. Within families and relationships, HIV could have a profound affect; sexual transmission or fears of sexual transmission could break up relationships and have an impact on new relationships. Fears of transmission to a baby could result in terminations of pregnancy and choices to not have children. Parenting was affected by HIV-related ill health: children caring for a sick parent, or parents caring for a sick child. Grief is more difficult and bereavement more profound when cause of death cannot be named and shared openly.
HIV is a medical condition that carries considerable, specific stigma, often because people lack information or they make moral judgements about how someone has contracted HIV. Stigma was attached to a misunderstood fear of casual transmission, but more by the concentration of HIV among those in society already stigmatised: gay men, sex workers, intravenous drug users. Stigma was fuelled by negative media coverage and resulted in people with HIV experiencing hostility, physical and/or verbal abuse, avoidance and exclusion. Although not as widespread, stigma and discrimination continue to have a detrimental impact on the lives of many people with HIV infection.
In a setting of stigma and discrimination, patients faced many challenging social and psychological issues. Before effective ART in the late 1990s, such challenges were dealt with alongside ongoing and deteriorating health problems.
Initial HIV infection may manifest as a seroconversion illness, usually a mild to severe flu-like condition (further details in Points 14.6, 14.13). The virus at this stage is rapidly replicating and infectivity is very high. Antibodies may not yet be present and the HIV test may be negative. This phase of the disease usually lasts for 2 to 4, but up to 12, weeks. This stage of HIV infection may pass unnoticed, especially if not associated with an awareness of infection risk. Individual HIV testing and a positive diagnosis may occur long after this phase of acute infection. Today with effective ARV treatment available at diagnosis, guidelines encourage HIV testing in all healthcare settings in order to reduce the proportion of individuals with such undiagnosed HIV infection.
In the presence of untreated HIV infection, the virus replicates within the CD4-carrying cells of the immune system – the T4 lymphocytes. The immune system is rendered increasingly weakened and becomes vulnerable to multiple opportunistic infections (OIs) – bacterial, fungal, viral and cancers. These organisms are present in the human body but become pathogenic in the presence of a weakened immune system.
Symptoms may develop during the 'latent' phase of HIV infection (CDC classification B) and may relate either to dysfunctional immune regulation or progressing immune deficiency. People with HIV infection may experience persistently swollen lymph nodes (persistent generalised lymphadenopathy/PGL) and unpleasant skin complaints. As the immune system declines, the initial symptoms of HIV infection represent the body's poor response to infections such as candida (thrush) or tuberculosis. In this situation, people with HIV infection may experience recurrent viral infections such as herpes and warts. Shingles may be the first indication that the immune system is impaired. Common bacterial pathogens frequently occur causing respiratory symptoms – difficulty breathing, cough, fevers, sweats – and gastrointestinal symptoms such as stomach cramps, diarrhoea and poor appetite. Such clinical features and symptoms – oral candidiasis (thrush), oral hairy leucoplakia and general symptoms such as fever, weight loss, fatigue, night sweats, and diarrhoea – predict the progression to AIDS. This phase was formerly called 'AIDS-related complex', or 'ARC'.
Prior to effective treatment and prophylaxis (preventative treatment) for opportunistic infections, pneumocystis carinii pneumonia (PCP) was the most common first AIDS-defining illness (with more advanced knowledge in 1999, the condition was renamed 'pneumocystis jiroveci'). Many people with HIV infection died as a result of PCP, which typically presented when the CD4 count reached around 200 cells/mm3. As effective treatment and prophylaxis for PCP was introduced, patients survived PCP but progressed to experience other opportunistic infections which appeared when the immune system became further weakened. Patients were living longer but facing more frequent and multiple debilitating illnesses. For as long as the immune system continued to deteriorate, death was inevitable.
In 1986, the Centers for Disease Control and Prevention (CDC) classified AIDS as the presence of one of 23 clinical conditions. The classification was revised in 199311 to emphasise the clinical importance of a CD4 T-lymphocyte count. An AIDS definition included all people with less than 200 CD4+ T-lymphocytes/uL. Three clinical conditions were added to the definition of AIDS in 1993: pulmonary tuberculosis, recurrent pneumonia and invasive cervical cancer.
Table 2: The symptoms caused by some of the major HIV-associated pathogens.
|
Candidiasis of oesophagus, bronchi, trachea, or lungs – commonly known as thrush |
A yeast infection causing pain, difficulty in swallowing and loss of appetite. |
|
Cryptococcal infection: a fungal infection. Most commonly causes meningitis |
Headaches, nausea, fever, fatigue, altered mental status, irritability and seizures. |
|
Cryptosporidiosis: a parasite in the gut |
Causes chronic diarrhea with frequent watery stools, stomach cramps, nausea, fatigue, weight loss, appetite loss, vomiting, dehydration and electrolyte imbalance. |
|
Cytomegalovirus (CMV) |
Eye infection: retinitis, causing blurry vision, loss of central vision and blindness. Gut infection: colitis, fevers, diarrhea and stomach pain. Oesophagitis: oesophageal ulcerations, severe pain and difficulty in swallowing. Lung infection: Pneumonitis pneumonia-like symptoms. |
|
Encephalitis infection of the brain |
Confusion, fever and tiredness. HIV encephalopathy is a direct result of HIV on the brain and has similar symptoms. |
|
Herpes simplex |
Recurrent very painful skin and mucous membrane. Ulceration can also cause bronchitis, pneumonitis, or esophagitis. |
|
Histoplasmosis: fungal infection |
Can cause pneumonia or disseminated infection. Leads to fever, fatigue, weight loss, difficulty in breathing, swollen lymph nodes and pneumonia-like symptoms. |
|
Kaposi's sarcoma (KS): a rare type of cancer of the lymph and blood vessels |
Red or dark purple lesions under the skin and living of mouth, nose, throat and other internal organs. Most common in people who acquired HIV sexually. |
|
Lymphoma |
Burkitt lymphoma, diffuse large B-cell lymphoma (DLBCL), primary central nervous system (CNS) lymphoma of brain. |
|
Mycobacterium avium intracellulare / complex (MAI/MAC): bacterial infection that occurs with very advanced immunosuppression |
Often disseminated infection. persistent fever, night sweats, fatigue, weight loss, anaemia, abdominal pain, dizziness, diarrhea and weakness. |
|
Mycobacterium tuberculosis: bacteria that causes tuberculosis |
TB can cause disease when there is only minimal immunosuppression and thus often appears early in the course of HIV infection. Occurs in the lungs and other body areas – malaise, night sweats, cough, fever, shortness of breath and weight loss. |
|
Pneumocystis jiroveci infection. (previously Pneumocystis carinii). Most commonly seen as a lung infection |
Often insidious onset over a period of weeks, with a prolonged period of increasing shortness of breath (usually on exertion), non-productive cough, fever and malaise, weight loss, night sweats and fatigue. |
|
Progressive multifocal leukoencephalopathy (PML) caused by Polyomavirus infection. Viral infection of the brain |
A progressive neurological and/or intellectual impairment, often including weakness or difficulties with speech, loss of coordination, difficulty walking, facial drooping, loss of vision, personality changes, difficulty speaking, muscle weakness. Usually inexorably progressive. |
|
Salmonella |
Recurrent bacterial infection from contaminated water and food – stomach cramps, bloody stools, diarrhoea, fever, headache, muscle pains, nausea. |
|
Toxoplasmosis: parasitic infection usually of the brain |
Causes multiple abscesses in the brain leading to altered mental state, confusion, delusional behaviour, severe headaches, fever, seizures and coma. Can affect eyes – pain and reduced vision. |
|
Wasting syndrome |
Cachexia, caused by HIV itself – progressive weight loss (>10%) weakness, fever, nutritional deficiency and diarrhoea. |
Multiple medications were used for the many opportunistic infections both to try to prevent them and as treatment as they appeared. However, until specific treatments were developed to prevent HIV replication and maintain the immune system, death from AIDS was almost inevitable. Most emerging treatments, particularly the early antiviral treatments, had unavoidable, often intolerable, side effects which required further medication to limit side effects and sustain treatment options. Multiple tablets and medications would need to be taken several times a day.
This appalling combination of frequent bouts of severe ill health, often different concurrent illnesses and ongoing treatments with many intolerable side effects that required additional medications, had an enormous effect on patients' mental health and impact on everyday life – at school or work, social life and relationships with friends, families and partners – and often caused disconnection, isolation, anger, fear, anxiety and depression.
It is often difficult to distinguish between the symptoms of the multitude of possible opportunistic infections, the symptoms which relate to treatment side effects, the symptoms resulting from the direct effect of HIV (for example, on the gastrointestinal system and on the brain) and the symptoms associated with severe psychological and mental health.
Research and treatment and related medical monitoring for HIV conditions progressed rapidly from the early 1980s until by 1996 effective treatment became available in the form of 'HAART' (highly active antiretroviral treatment). The symptoms associated with HIV progression changed as treatment and prophylaxis for opportunistic infections improved, and as research and development of effective and more tolerable antiretrovirals against HIV became increasingly available. Physical illnesses decreased and side effects were progressively better managed. For those who had not died of AIDS, there was a new sense of hope and less focus on inevitable death. However, 15 years living with HIV-related illnesses and treatments, and a belief of inevitable death, had left many with compromised health, low educational attainments, poor work and career progression, psychological and mental health issues, and low finances – all of which had to be reviewed in light of a future living rather than dying.
Before the introduction of effective therapy, HIV was a progressive, universally fatal infection. The development of HAART has transformed the clinical outcomes for people living with HIV, extending life expectancy towards that of the general population, reducing ill health and preventing HIV transmission. Today the aim is to maximise wellbeing and keep people in good health by diagnosing HIV promptly, starting treatment before symptoms or ill health develop, and preventing and treating other conditions associated with HIV. Today in the UK, symptomatic infection and opportunistic conditions in immunosuppressed patients are most commonly seen where ART is not available, or in people who are diagnosed late and have advanced infection.
There is still no cure for HIV and people live with a chronic, potentially infectious, stigmatising and sometimes unpredictable condition requiring daily medication for its control. Even with complete viral suppression, ART does not fully restore health, and treated infection is associated with a variety of complications and comorbidities, and non-AIDS complications, including cardiovascular disease, some cancers and impaired mental health. Measures of health-related quality of life are consistently lower amongst people living with HIV than in the general population.11,110,111
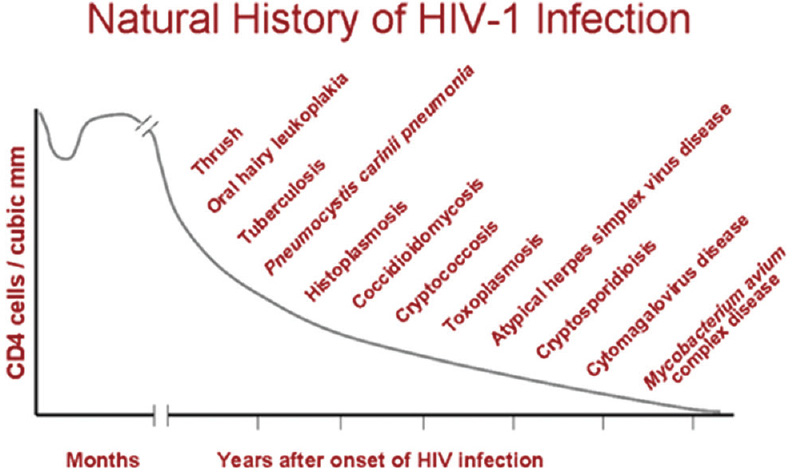
Figure 6: Opportunistic infections associated with advanced human immunodeficiency virus disease
Source: Microbiology Book, Natural History of HIV-1 Infection, accessed 28 January 2020, available at <http://www.microbiologybook.org/lecture/images/natural-history.gif>.
From the perspectives of the IBI, there are two major issues to consider. The first is the natural history of HIV drawn from publications in the early 1990s prior to effective combination antiretroviral therapy (ART). The second is to understand the major issues that are specifically relevant to young people growing up with HIV during decades of gradual improvement in available treatment.
Infants and very young children lack the full repertoire of mature immune responses that in adults can bring HIV replication under control following infection. Longitudinal studies of infants infected around the time of birth ('perinatally') demonstrated that HIV peaks in the circulation at very high levels (many millions of copies of HIV per ml of blood). The mature adult immune system typically brings the initial peak under control within three months, reducing the amount of HIV to below 100,000 c/ml, and frequently down to less than 10,000 c/ml. In contrast, young children were found to have a median (one measurement of average) of 100,000 c/ml two years after becoming infected perinatally.
The result of this impaired immune control, in the era before effective ART, was more rapid disease progression than in adult cohorts. Survival rates varied dramatically according to geography. In European and North American cohorts, 15% of perinatally infected children had died by age six years. In sub-Saharan African settings, only 15% were still alive at four years of age.
In all settings, about one fifth of the children had rapid disease progression, succumbing to pneumocystis pneumonia and other opportunistic infections within a few months of birth. Impaired growth and development were common features. HIV can penetrate and cause damage in the brain, with lifelong consequences if the damage occurs as the brain is rapidly growing, particularly in the first two years of life.
Even in high-income settings, children with perinatally acquired untreated HIV rarely developed cancers because they died of other causes – predominantly infections. If the cellular immune system was damaged at the time children first met common childhood infections such as chicken pox, the infection could disseminate and involve internal organs including the lungs and liver, resulting in death. This was in contrast to adults with HIV infection who would have had such infections in childhood and would have some degree of pre-existing immunity when exposed in later life.
In about one fifth of the children, a lung condition rarely described in adults, called 'lymphocytic interstitial pneumonitis', caused inflammatory changes throughout the lungs. Sometimes this was sufficiently severe to limit exercise tolerance and require treatment with corticosteroids (with attendant adverse effects). A more common problem was the association with chronic lung disease due to damage to the small airways (bronchiectasis). Such children would have very frequent bacterial chest infections, persistent productive cough, debility and increased risk of fatal bloodstream infections.
There are sparse data describing the natural history of HIV acquired at different ages during childhood beyond the perinatal period. It is known that the CD4 counts, which are naturally much higher in infancy, decline to around adult levels by age five years. Broadly, acquisition of HIV between 5 and 15 years of age was associated with better survival than acquisition in later life in haemophiliac populations, due to the co-morbidities of older people especially when co-infected with HBV or HCV. It can be construed that survival improves if HIV is acquired after infancy, as the cellular immune system matures, but detailed survival data have not been published.
As with adults, ART completely rewrote the natural history of HIV infection in terms of survival and quality of life for children with HIV infection. However, a fundamental difference for children throughout the early decades of the HIV epidemic was the lack of suitable antiretroviral drug formulations and dosing information. With the exception of didanosine, every ART drug was licensed for use in children years after licences for adults were granted. Younger children in particular were disadvantaged: efavirenz, for example, was never formulated for children age younger than three years despite being the first-line drug of choice for many years in adults. Most protease inhibitors required boosting with ritonavir that, as a suspension, tasted truly unpleasant. These issues did nothing to help long-term adherence: persuading children to take medicines was a daily, distressing battleground for many parents and caregivers.
Strategic clinical trials tended to follow adult trials so that progression from mono- to dual- to triple-ART as standard of care for children lagged a year or two behind that of adults. Options for second- and third-line treatments were limited, resulting in children staying on their first-line treatment longer. This, and the problems of poor adherence, resulted in the selection of high-level, multi-class resistance, especially in the cohort who started treatment in the early 1990s. Most young people who initiated HIV treatment after 1998 will have been started on triple ART, with much lower rates of acquisition of drug-resistant HIV.
For a graphic illustration of all the issues that young people have faced, the 12-minute film created by the Children's HIV Association using statements drawn directly from their experience is recommended: 'Life growing up'.112
Stigma, both overt and internalised, has been experienced by the majority of young people growing up with HIV. For their caregivers, the fear of causing distress led to well-meaning but misguided delays in letting many young people know that they were living with HIV. Discovering that you had a potentially sexually transmissible infection at the same time as going through adolescence and beginning to navigate towards first intimate relationships was devastating for many young people, leading to poor adjustment, immense anger and low self-esteem. In the past 20 years, healthcare professionals have been better at ensuring young people know the name of the virus ahead of entering puberty, typically by 11 years of age, but those infected in the 1980s and 1990s often found out in their mid-teens.
The long-term outcomes for young people surviving into adulthood are obviously not yet known. The survival estimates for people acquiring HIV mono-infection in adulthood have been published.113 Based on data from 18 European and North American cohorts comprising 88,504 patients initiating treatment between 1996 and 2010, a 20-year-old starting ART in the last of these time periods with a CD4 count above 350 was estimated to live to be 78 years. Given that young people infected during childhood would have been living with HIV for up to 20 years longer, it is a near-certainty that their life expectancy would be less than 78 years and possibly up to 20 years shorter. However, these crude estimates do not take into account advances in HIV therapy and other more broad medical interventions that will influence survival.
Data from the national UK/Ireland paediatric HIV cohort (CHIPS) were presented at the 10th International Workshop on HIV Pediatrics, Amsterdam, July 2018. Asad et al.114,115 reported that, of 474 young people with perinatally acquired HIV who transitioned at a median age of 18 years, 14 (3%) died, 36% experienced severe immunosuppression (CD4 <200 cells/mm3) and 46% experienced viral rebound (viral load >400 copies/ml on two consecutive samples) by six years into adult care. Of 387 with more complete clinical data, 27 (7%) had developed a new AIDS event at a median follow-up of 3.3 years. The cumulative incidence of mortality or a new AIDS event was 11% by six years.
In a smaller cohort from Imperial College Healthcare NHS Trust, London, UK, Foster et al. have reported that, of 180 young people with 921 person years of follow-up post transition, the all-cause mortality rate was 10-fold higher than in the age-matched general population. Seventeen (9%) developed a new AIDS-defining diagnosis, 20% had experienced anxiety and/or depression, 3 had attempted suicide and a further 4 had self-harmed.116
From the same cohort, extended to include 10–24-year-olds with perinatally acquired HIV, Chhabra et al. reported that 8/290 (2.8%) developed a malignancy. This represents a 13% higher incidence compared to the age-matched general population.117
Several studies have identified biomarkers and other measurements that predict a higher risk of cardiovascular disease in adolescents and young people with perinatally acquired HIV, although none have expressed the data as a quotable percentage risk of heart attacks or stroke. For example, the PositHIVe Health Study compared a cohort of 65 Brazilian children with HIV aged 8–15 years with 65 age- and sex-matched HIV-negative controls controls.118 The young people with HIV infection had higher inflammatory markers in their blood, higher levels of atherogenic lipids (fats), higher blood glucose levels and increased thickness of the carotid arterial walls, which are all potentially associated with premature atherosclerosis.
It is evident that living with perinatally acquired HIV mono-infection is statistically associated with higher morbidity and mortality than in the age-matched general population. In the context of dual infections with either hepatitis B or hepatitis C, plus morbidities associated with the underlying condition for which blood products were required, both survival and quality of life are affected.
Since zidovudine was first licensed in 1987 for the treatment of patients with symptomatic disease, there have been more than 25 antiretroviral drugs licensed for the treatment of HIV-positive adults. Combination antiretroviral therapy (ART; combinations of multiple drugs together) has resulted in marked clinical improvements in the treatment of patients with HIV infection, with some now experiencing near normal life expectancy and many now living well with HIV infection. This section describes the different antiretroviral drug classes, the key landmark studies of ART, current standard of care, when to start ART and drug resistance. It may be helpful to review Figure 1b: The HIV Lifecycle. The following section (14.11) will address in more detail the effectiveness of ART regimens and associated side effects.
Nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) are competitive inhibitors of the viral enzyme reverse transcriptase which is responsible for constructing a proviral DNA copy from the single-strand viral RNA (the genetic code of the HIV virus). A proviral DNA copy is required to allow integration of the viral genome into the host cell (CD4+ T helper lymphocyte). Inhibition of viral reverse transcriptase stops the viral replication cycle and thus suppresses viral replication.
The first NRTI to be given regulatory approval and made available to treat patients with HIV/AIDS was zidovudine in mid-1987. The first-generation NRTIs (zidovudine, didanosine, zalcitabine and stavudine) were associated with more severe and often long-lasting side effects including anaemia, nausea, peripheral neuropathy, liver disease and peripheral lipoatrophy. The later NRTIs (lamivudine, abacavir, emtricitabine, tenofovir DF and tenofovir AF) are more tolerable with fewer side effects. Today, a combination of two NRTIs (usually lamivudine and abacavir or emtricitabine and tenofovir DF/AF) forms the backbone of highly active ART regimens which are the current standard of care for treatment of adults with HIV infection.
Non-nucleoside reverse transcriptase inhibitors (NNRTIs) are non-competitive inhibitors of the viral reverse transcriptase enzyme and act by blocking the action of the enzyme in constructing a proviral DNA copy from the single-strand HIV viral RNA. This stops the viral replication cycle and suppresses viral replication.
NNRTIs are one of the options for the third antiretroviral drug in combination with two NRTIs to form triple combination ART. The first NNRTI to receive regulatory approval was nevirapine in early 1998. There are currently five NNRTIs licensed for treatment of HIV infection as part of combination ART. The last to receive approval was Doravirine in 2019. The NNRTIs have been associated with rashes, liver toxicity and neuropsychiatric side effects including sleeping difficulties.
Protease inhibitors (PIs) are competitive inhibitors of the viral enzyme protease. The protease enzyme is responsible for the breakdown of large polyproteins into smaller proteins required for the assembly of new mature viral particles. New immature HIV virions can be produced in the presence of protease inhibitors, but they are unable to infect new cells, in effect blocking the HIV replication cycle, suppressing viral replication.
PIs are used in combination with other antiretroviral drugs, usually two NRTIs, to form triple combination ART. The first PI to receive regulatory approval was ritonavir in August 1996. Although shown to have some clinical benefit in randomised trials, ritonavir was not well tolerated and was quickly superseded by other PIs. Ritonavir is a potent inhibitor of certain liver enzymes and is now only used in lower doses as a pharmacological booster to other protease inhibitors (for example, darunavir, atazanvir). The earlier protease inhibitors (indinavir, nelfinavir, saquinavir and lopinavir) were associated with significant side effects including chronic diarrhoea, nausea and renal stones.
Integrase inhibitors (INIs) block the activity of the viral enzyme integrase. The integrase enzyme is responsible for the insertion of the HIV proviral DNA into the host cell chromosome which, following cell activation, enables new viral particles to be produced. INIs thus block the replication cycle of HIV, suppressing viral replication.
The first INI to receive regulatory approval was raltegravir in December 2007, the most recent bictegravir (as part of a fixed dose combination tablet) in June 2018. In general, INIs are well tolerated with fewer people discontinuing them due to side effects compared to other antiretroviral drugs. They are used in combination with other antiretroviral drugs, usually two NRTIs, to form triple combination antiretroviral therapy.
Entry inhibitors stop the virus from entering its target cell. There are two types of entry inhibitors, which currently have regulatory approval for treatment of HIV infection. Enfuvirtide is a fusion inhibitor and received regulatory approval in May 2003. It is given by subcutaneous injection and has mainly been used to treat people with extensive antiretroviral treatment experience with few treatment options due to drug resistance.
Maraviroc blocks the virus from binding to a co-receptor molecule (CCR5) on the surface of the target cell, thus stopping the virus from gaining entry into the cell. It is not commonly used, as it has been shown to be less effective in combination ART compared to other antiretroviral drugs.
The British HIV Association (BHIVA) clinical guidelines for treatment of adults with HIV infection with ART outline best clinical practice in the UK. Recommendations are based on evidence from clinical studies including randomised clinical trials. BHIVA guidelines are accepted by healthcare professionals providing treatment and care for HIV-positive patients in the UK as the national reference document for good clinical practice. The process by which BHIVA produces its clinical guidelines has since 2012 been approved by the National Institute for Health and Care Excellence (NICE).
The BHIVA ART guidelines were first published in April 1997 and recommended starting ART with at least dual NRTI therapy. The guidelines were updated in July 1998 and recommended HAART for all patients with two NRTIs and either an NNRTI or a PI. The guidelines have since been updated at regular intervals to take into account new evidence of best practice. The last revision was published in 2016 with an interim statement in 2019. 10 Other international guidelines which help to inform best practice include those from the European Clinical AIDS Society (EACS) and from the International AIDS Society (USA).
The current BHIVA-recommended regimens (preferred and alternative) are detailed in Table 5 and are largely similar to other international clinical guidelines. Patients newly diagnosed with HIV infection are currently most commonly started on a regimen containing two NRTIs (emtricitabine and tenofovir DF/Tenofovir AF or lamivudine and abacavir) and an INI due to fewer side effects, better tolerability and higher virological efficacy compared to other regimens. Treatment regimens for ART-experienced patients will depend on previous intolerance and virological failure to antiretroviral drugs, and the presence of HIV drug resistance.
The early trials of ART largely investigated efficacy in patients with AIDS or non-AIDS symptomatic disease with low CD4 counts as these patients were most in need of effective therapy. Compared to patients with asymptomatic disease and higher CD4 counts, those with symptomatic disease had significantly higher rates of clinical disease progression and death. Once the CD4 count falls to below 200 cell/uL, the risk of clinical disease progression increases rapidly. For this reason and because of the risk of drug side effects and treatment emergent drug resistance, earlier versions of the BHIVA guidelines recommended starting therapy before the development of significant immune deficiency, at a CD4 count of between 200 and 300 cell/uL, later increased to around a CD4 count of 350 cell/uL. These recommendations were largely based on expert opinion, an understanding of risk of clinical disease progression, and the known efficacy and safety of ART at the time.
Previously, trials of zidovudine monotherapy, first reported in 1993, had shown no difference in survival or progression to AIDS at three and five years of follow-up in patients who started therapy immediately, compared to deferring starting therapy till they had developed symptomatic disease or when their CD4 count had fallen. Although immediate treatment with zidovudine did result in clinical benefit, this was short term and time limited. There was no difference in survival rates for both treatment strategies at three years of follow-up.
It was not until 2015 that definitive evidence was first presented that immediate treatment with combination ART was associated with clinical benefit, irrespective of baseline CD4 count. In this study, patients with asymptomatic disease and a CD4 count above 500 cell/uL were randomised to either start ART immediately or defer till their CD4 counts had fallen towards 350 cell/uL or they had developed symptomatic disease. The study showed that even in this population, who had low risk of clinical progression, starting ART immediately resulted in a greater than 50% reduction in clinical progression to either any serious AIDS-related event, serious non-AIDS-related event or death from any cause compared to the deferred therapy group. The primary end point occurred in 1.8% (0.60 events per 100 person years) in the immediate group versus 4.1% (1.38 events per 100 person years) in the deferred group. Clinical benefit was seen across all strata of CD4 counts. Importantly, risk of adverse events was no different between the two groups, establishing the safety of starting ART in early disease.
As a result of these study findings, BHIVA updated their treatment guidelines in 201573 and recommended that patients with HIV start ART irrespective of CD4 count. They had previously recommended in 2013 that patients with a risk of onward transmission of HIV should consider starting ART, as treatment as prevention, irrespective of CD4 count. In April 2018, NHS England published a clinical commissioning policy ('Immediate ART') stating that all patients with HIV infection should be recommended to start ART irrespective of CD4 count or risk of onward transmission.
Although starting ART in early disease is preferable, many patients are diagnosed late when their CD4 counts have already fallen to <350 cell/uL. In 2018, 43% of patients newly diagnosed had a CD4 count of 350 cell/uL or less. The proportion was higher in certain sub-populations such as black African adults and those aged 50 years of age or older.
In the early clinical trials of mono and dual NRTI therapy, clinical benefit was shown to be time limited. Viral isolates grown from blood taken from patients who had been treated for more than six months of zidovudine were shown to have markedly reduced susceptibility to the antiviral effect of zidovudine in vitro. Subsequent tests showed that these viral isolates had multiple mutations in the viral gene responsible for the coding of viral enzyme reverse transcriptase.124 These viral mutations conferred high-level resistance to zidovudine.
In patients treated with mono or dual NRTI therapy, the viral load in blood initially falls as a consequence of the antiviral effect of the drugs but, then after a few months or more, starts to increase back to pre-treatment levels. This rise in viral load is temporally associated with the emergence of mutations in the viral genome, which results in viral resistance to the action of the drugs. Virological failure and the emergence of drug resistance are secondary to inadequate virological potency and the failure to suppress viral load to undetectable levels. HAART is more effective as it usually results in optimal suppression of viral replication and thus viral load which, as long as the patient is adherent to therapy, is maintained over time, preventing the emergence of drug resistance.
Viral mutations causing drug resistance have been identified with all currently licensed antiretroviral drugs. With some drugs, a single viral mutation causes high fold resistance (low genetic barrier – for example, lamivudine, nevirapine), whilst with other drugs the accumulation of multiple mutations over time is required or the development of resistance mutations is rare (high genetic barrier – for example, boosted PIs, dolutegravir, bictegravir). Viral mutations are associated with cross-resistance to other drugs in the same class and remain present in HIV-infected long-lived T lymphocytes. If patients are re-exposed to the same drugs, then these mutations can quickly re-emerge. Viral mutations can also be transmitted. In patients newly diagnosed with HIV in the UK, approximately 8% have transmitted drug resistance.
Many patients who were treated with mono or dual NRTI therapy and the earlier HAART regimens experienced virological failure and developed resistance to one or more classes of antiretroviral drugs. In these treatment-experienced patients, treatment options were limited due to drug resistance. However, with the advent of INIs and the newer boosted PIs with high genetic barriers, nearly all treatment-experienced patients with previous drug resistance can be treated with effective antiretroviral regimens. The main factor associated with virological failure and emergence of drug resistance viral mutations on HAART regimens is non-adherence to therapy.
With the current recommended ART regimens, efficacy and tolerability are high. As long as patients are adherent to their medication, then virological failure and emergence of drug resistance are very uncommon.125
Predictive factors of treatment outcome include both HIV- and patient-related factors. HIV-related factors include baseline CD4 count and viral load, drug resistance and stage of disease.
Patients with high baseline viral load (>100,000 copies/ml), low CD4 counts (<200 cell/uL) and symptomatic disease, particularly a previous AIDS diagnosis, have had in general poorer treatment outcomes compared to patients starting ART with lower viral loads, higher CD4 counts and in early disease. The efficacy of ART in terms of viral suppression has frequently been reported to be lower in patients with high baseline viral loads, this is largely related to the longer time for plasma viral load to fall to less than <50 copies/ml and the virological potency of antiretroviral regimen. However, with the more modern ART regimens which include an INI, rates of viral load suppression are similar for patients with a baseline viral load above 100,000 copies/ml or a CD4 count below 200 cell/uL.
Patients who start ART in advanced disease, with CD4 counts less than 200 cell/uL or lower, continue to have a higher risk of new AIDS diagnosis or death for up to two to three years from the start of ART than patients starting ART with a CD4 count >350cell/uL.
The incidence of side effects and tolerability of ART regimens, particularly the older ART regimens, is higher in patients with advanced disease including AIDS. This is largely related to the fact that they are unwell with symptomatic disease and have a more limited capacity to tolerate side effects. However, the tolerability of INIs containing regimens is high, even in patients with AIDS and severe immune deficiency or those requiring chemotherapy.
Of demographic factors, only older age has been consistently associated with poorer treatment outcomes and this is likely to be related to high risk of disease progression and a higher prevalence of non-AIDS comorbidities. Neither gender nor race are generally associated with poorer outcomes if access to care and tolerability of regimens are taken into account.
The most important patient-related factor predictive of treatment outcome is adherence. Non-adherence to ART is associated with treatment failure and emergence of HIV drug resistance. In general, high levels of adherence to a treatment regimen (>90%) are required to maintain virological suppression, though this may vary between different regimens. Factors that have an impact on ability to adhere to therapy includes the number of pills and dosing frequency of ART regimens, side effects of medication, mental health problems such as depression and anxiety, social welfare factors such as housing and financial difficulties, and co-morbidities. Supporting patients to adhere to their ART regimens is an essential part of HIV care. Fewer pills, once-daily dosing and improved tolerability of current ART regimens have helped to improve adherence.
If patients stop therapy, then blood viral load rebounds towards pre-treatment levels within one month of therapy discontinuation. ART unfortunately does not eliminate HIV infection: it very effectively reduces HIV replication to very low levels and maintains effective inhibition of viral replication over time, allowing reconstitution of the immune system. Once therapy is stopped, viral replication increases to pre-treatment levels, causing progressive damage to the immune system and ultimately severe immune deficiency and the clinical disease associated with this. Usually the CD4 count will fall to pre-treatment levels within six months of stopping ART and will continue to fall unless ART is restarted. For these reasons, ART needs to be taken lifelong. There is a significant amount of ongoing research, investigating HIV cure and eradication strategies.
The effectiveness of early mono and dual therapy regimens was low and time limited. In the first randomised clinical trial of zidovudine monotherapy (reported in 1987) in patients who had advanced disease with AIDS or non-AIDS symptomatic disease, at a median of four months of follow-up <1% of patients who received zidovudine had died compared to 14% of patients who received placebo.32 However, this clinical benefit was not sustained and mortality remained high in clinic populations. Treatment benefit was limited by side effects and the development of drug resistance.
Randomised clinical end point trials of dual therapy (first reported in 1995) compared zidovudine monotherapy versus a combination of zidovudine with either didanosine or zalcitabine and showed a relative reduction in mortality of between 30% and 40% with dual therapy compared to zidovudine alone over a median of 30 months' follow-up. Clinical benefit was sustained out to two to three years of treatment.67 Despite this improvement, mortality in this patient population with advanced disease remained high with approximately one in five patients who received dual therapy dying over the duration of the study. A further one in three patients experienced clinical disease progression.
It was not until the development of HAART (first reported in 1996) did we see sustained virological suppression and improvements in the immune system associated with long-term clinical benefit. The advent of HAART had a major impact on reducing mortality and clinical progression. The development of new classes of ART, the PIs and the NNRTIs, allowed the investigation of triple combination therapy regimens with two NRTIs in combination with a third agent, either a PI or a NNRTI. Studies of the PI indinavir in combination with lamivudine and either zidovudine or stavudine showed improved survival and reduced clinical progression compared to dual therapy. Higher proportion of patients achieved virological suppression at one year on triple therapy compared to dual therapy.49
These clinical end point studies also established that changes in plasma viral load and CD4 count were predictive of clinical outcome. Thus, in subsequent clinical trials, changes in these surrogate markers were used to assess efficacy of different regimens. Rises in CD4 count and proportion of patients achieving virological suppression at 48 and 96 weeks became standard outcome measures for investigating efficacy of new antiretroviral drugs and regimens.
Over the next 10 to 15 years with the development of newer PIs (used with ritonavir as a booster) and newer NNRTIs, the efficacy, safety and long-term tolerability of HAART regimens continued to improve significantly. In 1998, a study of a regimen containing efavirenz, zidovudine and lamivudine reported that 64% of patients achieved an undetectable viral load of <50 copies/ml at 48 weeks of therapy. Similar results were reported in 2002 with a combination of lopinavir (boosted with ritonavir), stavudine and lamivudine. The boosted PIs were also associated with a lower risk of HIV drug resistance at virological failure.
A large randomised clinical trial comparing efavirenz versus lopinavir/ritonavir each in combination with two NRTIs reported (in 2008) a longer time to virological failure with the efavirenz containing regimen. At 96 weeks, the proportion of patients with a viral load <50 copies/ml was 89% with the efavirenz containing regimens versus 77% with lopinavir/ritonavir.125 This established efavirenz in combination with two NRTIs as the standard of care for several years after.
Abacavir and tenofovir DF emerged as replacements for stavudine and zidovudine due to better tolerability and safety profiles. Abacavir in combination with lamivudine and tenofovir DF (or tenofovir AF) with emtricitabine continue to be recommended as NRTI backbones in combination with a third agent in current clinical guidelines.
Raltegravir was the first INI to be licensed in 2007 and, together with the newer PI darunavir (boosted with ritonavir), significantly improved the treatment outcome of patients who had experienced virological failure on previous regimens and had developed dual or triple class HIV drug resistance. These patients previously had limited treatment options. Now most ART-experienced patients with history of virological failure, drug resistance and/or treatment intolerance can be treated with effective combination regimens.
INIs have been shown to have similar virological efficacy to PIs or efavirenz containing triple regimens but overall are more effective due to better tolerability and lower discontinuation rates from adverse events. In 2013, a combination of dolutegravir, abacavir and lamivudine was shown to have significantly higher rates of viral suppression compared to a combination of efavirenz, tenofovir DF and emtricitabine (89% versus 81% at 96 weeks).122 The difference was largely attributable to a lower discontinuation rate secondary to adverse events in the dolutegravir arm (2%) compared to the efavirenz arm (10%). In national and international clinical guidelines, INIs containing combination regimens have largely become the preferred treatment of choice in patients starting ART for the first time due to higher virological efficacy and tolerability compared to other regimens.
With the current recommended antiretroviral regimens, patients who adhere to their therapy can expect to experience sustained virological suppression with undetectable viral loads in blood with limited risk of virological failure and emergent drug resistance. Data from Public Health England show that, in the UK in 2018, 97% of patients receiving HIV care were on antiretroviral therapy of whom 97% had undetectable viral loads. Sustained virological suppression and immune reconstitution has resulted in marked improvements in life expectancy and reduction in HIV-associated co-morbidities, with reports of near normal life expectancy in patients starting therapy in early disease. Further data on life expectancy is outlined in Point 14.14.
However, despite these excellent treatment outcomes, people living long term with HIV infection on ART experience an increased prevalence of non-AIDS comorbidities, including those associated with ageing such as cardiovascular and chronic kidney disease and an increased incidence of certain cancers (such as lymphoma and lung cancer) compared to an aged matched HIV negative population. Quality of life is frequently impacted by a higher prevalence of mental health disorders, chronic musculoskeletal symptoms, metabolic disorders and frailty.
The earlier antiretroviral drugs were associated with significant short- and long-term side effects, often treatment limiting or frequently having an impact on quality of life. The tolerability and dosing convenience of ART regimens have significantly improved over time with much lower rates of discontinuation due to adverse events. The reported efficacy and adherence to current recommended treatment regimens are high. However, like all medication, side effects do occur with all antiretroviral drugs and can affect tolerability and have an impact on quality of life.
Common side effects (as defined by a prevalence of greater than 1/100 patients) associated with individual drugs are listed in Table 4.
Common short-term side effects, mainly with the older antiretroviral drugs, include: gastrointestinal symptoms (nausea, vomiting, diarrhoea), hypersensitivity reactions (fever and rash), neuropsychiatric (insomnia, abnormal dreams, low mood, anxiety, headaches), hepatitis (abnormal liver function), asthenia and fatigue. Some of these would have been severe enough to cause treatment discontinuation; others contributed to low-grade symptoms which had a negative impact on quality of life.
One potential adverse effect of ART is immune reconstitution inflammatory syndrome (IRIS) which, although not a direct effect of drugs, is a consequence of HIV infection mainly seen in patients with AIDS and severe immune deficiency starting ART. In advanced HIV disease, the ability of the immune system to effectively fight infections is lost. Infections that have previously been kept in check through immune surveillance re-activate and cause disease. Treatment with ART results in reconstitution of the immune system allowing the immune system to once again recognise and fight infection. The clinical impact of this is that patients may become ill (occasionally severely or to the point where life is threatened) within a few weeks of starting ART with symptoms of an opportunistic infection (for example, mycobacterial disease) which they may have previously been treated for or had been undiagnosed and are asymptomatic from. With continued treatment with ART, IRIS generally resolves but can cause quite severe ill health at the time. Approximately 10% of patients with very advanced disease (CD4<50 cell/uL) develop IRIS within the first few weeks of starting ART.
Several severe and frequently disabling side effects have been associated with long-term use of ART, most commonly with the first-generation antiretroviral drugs.
Lipodystrophy, a fat redistribution disorder which included peripheral lipoatrophy and lipohypertrophy was commonly seen with the older ART regimens. Peripheral lipoatrophy (loss of subcutaneous fat) was common with the first-generation NRTIs (zidovudine, stavudine, zalcitabine and didanosine). This particularly affected the face and limbs and resulted in disfigurement, stigma and anxiety. Lipoatrophy improves on discontinuation of these NRTIs, but many patients exposed to these drugs in the past continue to require treatment with facial fillers at regular intervals to help improve psychological and physical wellbeing.
Lipohypertrophy (fat accumulation) usually presented with increased visceral fat in the abdomen resulting in abdominal distension and swelling. It is also associated with fat accumulation at the base of the neck (buffalo hump) resulting in disfigurement. Lipohypertrophy was largely reported with the first-generation PIs such as indinavir. Aetiology, however, is complex and discontinuation of the PI does not result in improvement. Increased visceral fat is often seen in the general population with ageing. It is generally not seen with the newer PIs atazanavir and darunavir. Increased visceral fat is associated with increased risk of diabetes and liver disease.
Peripheral neuropathy is a complication of HIV infection and usually presents as numbness and pins and needles in the feet and lower legs and largely improves over time with treatment of HIV infection. However, some of the earlier NRTIs also caused a drug-associated peripheral neuropathy which was frequently painful and difficult to treat. Despite discontinuation of the specific NRTI, symptoms often persisted for many months and years.
Other conditions associated with long-term treatment with ARVs include chronic liver disease, exocrine pancreatic deficiency, low bone mineral density and chronic kidney disease. Some of these have been associated with significant disability and chronic symptoms.
Nearly all the examples of side effects of treatment described in witness statements can be found in the list of common side effects in Table 4 and/or have been described earlier. There is no doubt that in many patients side effects from medication, particularly from the older ART regimens, significantly affected their quality of life on a daily basis. However, it should also be noted that many of these symptoms may also be associated with symptomatic HIV disease. Constitutional symptoms, such as weight loss, diarrhoea, poor appetite and fatigue, are common in patients presenting with symptomatic disease. Peripheral neuropathy, dermatitis, blood disorders such as anaemia, gingivitis and periodontal disease, and memory difficulties are well-recognised complications of symptomatic HIV infection. These problems would generally resolve with time with effective ART and improvement of the immune system. Those symptoms occurring for the first time or worsening on starting ART would more likely to be side effects from medication.
Although side effects are common, the long-term benefit of antiretroviral therapy in terms of improved survival and decreased HV associated co-morbidities generally outweighs potential harm. In 2006, a large randomised trial comparing a treatment strategy of continued use of ART to maintain viral suppression with a strategy of episodic use of ART, guided by CD4 count (drug conservation arm), reported better clinical outcomes with continued viral suppression.121 Rates of death from any cause and new opportunistic disease were significantly lower in the viral suppression arm and, importantly, there was no difference in the rate of adverse events between the two treatment strategies. In addition, continued viral suppression reduced the incidence of major non-HIV-related co-morbidities (cardiovascular, renal and hepatic diseases). The study established the importance of continued viral suppression from ART in reducing both HIV and non-HIV-associated clinical disease. These results do not, however, minimise the impact treatment-related side effects can have on quality of life. Many trials of ART have not reported on quality of life outcomes. Although long-term treatment with ART is essential in improving survival and preventing disease progression, maintaining good quality of life is equally important.
The more modern antiretroviral regimens have good tolerability, low rates of discontinuation (<5%) due to adverse events at one year of treatment and few known long-term side effects. Efficacy in terms of virological suppression at one year is high (>90%).
Table 3: BHIVA guidelines: summary recommendations for choice of ART 201673
|
Preferred |
Alternative |
|
|
NRTI backbone |
Tenofovir DF and emtricitabine Tenofovir AF and emtricitabine |
Abacavir and Lamivudine |
|
Third agent (alphabetical order) |
Atazanvir/r /r: boosted with ritonavir |
Efavirenz |
Table 4: Common side effects associated with antiretroviral drugs
Table note: The list has been sourced from the summary of product characteristics for each antiretroviral drug. The list is not exhaustive but reflects those symptoms commonly reported in clinical trials or clinic populations. Enfuvirtide, maraviroc and tipranavir have not been included as they have been used infrequently.
|
Antiretroviral drug |
Regulatory approval |
Common side effects (>1/100 persons) |
Comment |
|
Nucleoside/nucleotide reverse transcriptase inhibitors |
|||
|
Zidovudine |
March 1987 |
Blood disorders: anaemia, neutropaenia Nausea, vomiting, diarrhoea, abdominal pain Headache, malaise, dizziness Myalgia Lipoatrophy (subcutaneous fat loss) |
Side effects were common and often treatment limiting, particularly with the higher dose earlier treatment schedules. Lipoatrophy was common with long term use. |
|
Didanosine |
June 1992 |
Lipoatrophy Peripheral neuropathy Headache Nausea, diarrhoea, abdominal pain, flatulence, dry mouth Hepatitis Fatigue, asthenia Rash Joint and muscle pain |
Earlier formulations of didanosine were very commonly associated with gastrointestinal side effects and frequently treatment limiting. Painful peripheral neuropathy was very common with long term use. Pancreatitis and severe liver disease (including non-cirrhotic portal hypertension) have been associated with long-term use. |
|
Zalcitabine |
September 1994 Withdrawn December 2006 |
Peripheral neuropathy Headaches, dizziness Nausea, vomiting, abdominal pain, oral ulcers Pancreatitis |
Painful peripheral neuropathy was a common problem on long-term use and symptoms persisted long time after discontinuation. Pancreatitis and liver disease have been associated with long term use. |
|
Stavudine |
May 1996 |
Lipoatrophy Peripheral neuropathy Insomnia, headache Nausea, diarrhoea, abdominal pain Rash |
Lipoatrophy and painful peripheral neuropathy were very common with long term treatment with stavudine. Severe pancreatic and liver disease have been associated with long term use. |
|
Lamivudine |
August 1996 |
Insomnia, headaches Nausea, diarrhoea, abdominal pain Rash, hair loss Joint and muscle pain Cough, nasal symptoms |
Rates of drug discontinuation are low. Long term treatment is generally well tolerated. |
|
Abacavir |
July 1999 |
Hypersensitivity reaction (rash, fever, systemic symptoms) Nausea, vomiting, diarrhoea, poor appetite Headache Lethargy, fatigue |
Gastrointestinal side effects usually occur in the first few weeks of treatment and often then settle.. Abacavir is generally well tolerated in the long term but has been associated with increased risk of cardiovascular disease. |
|
Tenofovir disoproxil |
February 2002 |
Headache, dizziness Nausea, vomiting, diarrhoea, abdominal pain, flatulence Hypophosphataemia Rash Fatigue, asthenia |
Long term use is associated with renal toxicity (proximal renal tubulopathy), loss of bone mineral density, and increased risk of chronic kidney disease. |
|
Emtricitabine |
October 2003 |
Allergic reaction. Insomnia, headaches, dizziness Nausea, diarrhoea, abdominal pain Skin pigmentation Hepatitis |
Long term treatment is generally well tolerated. |
|
Tenofovir Alafenamide |
April 2016 |
Nausea, vomiting, diarrhoea, abdominal pain, flatulence Headache, dizziness, abnormal dreams Rash Fatigue |
Only available in a co-formulation tablet with other antiretroviral drugs. Compared to tenofovir DF, tenofovir AF has not been associated with loss of bone mineral density and renal toxicity is rare. Long term tolerability is generally good. |
|
Non-nucleoside reverse transcriptase inhibitors |
|||
|
Nevirapine |
February 1998 |
Hypersensitivity reaction (fever, rash, hepatitis) Headache Nausea, vomiting, diarrhoea Hepatitis. |
In the first few weeks of treatment severe life threatening skin reactions and hepatitis have occurred. In the long term nevirapine is well tolerated with few side effects. |
|
Efavirenz |
May 1999 |
Insomnia, abnormal dreams, anxiety, depression Headache, dizziness, somnolence Nausea, vomiting, diarrhoea Transaminitis (abnormal liver function tests) Rash Fatigue |
More than any other antiretroviral drug, neuropsychiatric symptoms were common with efavirenz and were the main reason for treatment discontinuation over time. Increased risk of suicidality has been reported. Gynaecomastia reported with long-term use. |
|
Etravirine |
August 2008 |
Rash, hypersensitivity reaction Sleep disorders, insomnia, anxiety Headache Transaminitis (abnormal liver function tests) |
Safety profile similar to other NNRTIs within class. Neuropsychiatric symptoms less common. |
|
Rilpivirine |
November 2011 |
Insomnia, abnormal dreams, depression Headache, dizziness Nausea Rash Transaminitis |
Generally well tolerated with long term use with low discontinuation rate due to adverse events. Incidence and grade of neuropsychiatric symptoms much lower compared to other NNRTIs. |
|
Protease inhibitors |
|||
|
Ritonavir |
August 1996 |
Nausea, vomiting, diarrhoea, abdominal distension, flatulence, abdominal pain Hyperlipidaemia Lipodystrophy Oral and peripheral paraesthesia Hepatitis Headache, dizziness, rash |
Ritonavir was first licensed as an antiretroviral drug, but intolerable gastrointestinal side effects at treatment doses limited use. Has subsequently been used as a pharmacokinetic booster for other PIs. |
|
Indinavir |
October 1996 |
Headaches, dizziness, insomnia Nausea, vomiting, diarrhoea, dry mouth Hyperbilirubinaemia, abnormal liver function Lipodystrophy Renal stones (associated with renal impairment) Fatigue, asthenia Rash, dry skin Muscle pain Hyperlipidaemia |
Indinavir was first used without ritonavir boosting and was difficult to adhere to, in view of its dosing schedule. Renal stones and chronic kidney disease were common with long term use. Central adiposity was a common and disfiguring long term side effect. Increased risk of bleeding reported in haemophiliacs No longer recommended in guidelines |
|
Saquinavir |
October 1996 |
Nausea, vomiting, diarrhoea, abdominal distension Transaminitis (abnormal liver function tests) Headache, dizziness Hyperlipidaemia Lipodystrophy |
Saquinavir was first used with ritonavir as a booster and had a high pill burden. Like all first generation PIs gastrointestinal side effects were very common and often led to drug discontinuation. Increased risk of bleeding reported in haemophiliacs No longer recommended in guidelines |
|
Nelfinavir |
January 1998 Withdrawn 2013. |
Nausea, vomiting, diarrhoea, flatulence Rash Hyperlipidaemia Lipodystrophy Transaminitis (abnormal liver function tests) |
Gastrointestinal side effects were very common, with up to 50% of patients experiencing diarrhoea. Nelfinavir was withdrawn in 2013. |
|
Lopinavir |
March 2001 |
Nausea, vomiting, diarrhoea, abdominal distension, abdominal pain Hyperlipidaemia Hepatitis Rash, hypersensitivity reactions Headache, dizziness Lipodystrophy |
Gastrointestinal symptoms were very common in particular diarrhoea. Has largely been replaced by atazanavir and darunavir. |
|
Atazanavir |
March 2004 |
Hyperbiirubinaemia, jaundice Rash Nausea, diarrhoea, abdominal pain Headache |
Long term use associated with renal stones and increased risk of chronic kidney disease. Lower level of gastrointestinal symptoms compared to earlier PIs. |
|
Fosamprenavir |
July 2004 |
Nausea, vomiting, diarrhoea Rash Hyperlipidaemia Headache Transaminitis |
As with other PIs gastrointestinal side effects were common. No longer recommended in guidelines |
|
Darunavir |
February 2007 |
Nausea, diarrhoea, abdominal pain, abdominal distension Rash, hypersensitivity Transamnitis (abnormal liver function tests) Headache, dizziness |
Currently most common PI used in ART treatment regimens. Generally well tolerated in the long term, low grade gastrointestinal side effects may occur. |
|
Integrase inhibitors |
|||
|
Raltegravir |
December 2007 |
Insomnia, abnormal dreams, depression Headaches dizziness, vertigo Rash Loss of appetite, nausea, diarrhoea Tiredness |
Severity of side effects is generally low and frequently settle in the first few weeks of treatment. Discontinuation rates are low. |
|
Elvitegravir |
May 2013 |
Abnormal dreams Nausea, diarrhoea, Headache, dizziness Rash |
Only available in co-formulated tablet with cobicistat, emtricitabine and tenofovir DF/AF. Generally well tolerated with low discontinuation rates, though use has been more limited compared to other integrase inhibitors. |
|
Dolutegravir |
January 2014 |
Insomnia, abnormal dreams, depression, anxiety Headache, dizziness Nausea, diarrhoea Rash |
Generally well tolerated in the long term, though neuropsychiatric side effects have emerged as a problem with some patients. |
|
Bictegravir |
June 2018 |
Abnormal dreams, depression Headache, dizziness Nausea, diarrhoea |
Only available in co-formulated tablet with emtricitabine and tenofovir AF. Well tolerated in clinical trials with low discontinuation rate from adverse events reported. Data on long term tolerability in clinic populations is limited. |
The general aspects of prognosis and life expectancy have been summarised above under Point 14.3. In summary, an average of 8 to 10 years pass, in the natural course of HIV following initial infection, before AIDS-defining events occur. Without antiretroviral therapy (ART), usually these illnesses lead to death within two years. Factors which drive disease progression as outlined above are level of HIV-viremia and age. The introduction of highly active ART in 1996 was able to dramatically change life expectancy in the setting of achieving persistent suppression of viral replication.49 With the introduction of better tolerated drugs, introduction of longer drug half-lives and fixed dose formulations, life expectancy has continued to increase substantially. If ART is started early enough, life expectancy approaches that of the general population as demonstrated in the Swiss cohort.126 This was also demonstrated in the Antiretroviral Therapy Cohort Collaboration. In their analysis, even in the late ART era, survival during the first three years of ART continues to improve, which probably reflects transition to less toxic antiretroviral drugs, improved adherence, prophylactic measures and management of comorbidity.113
(a) Does early diagnosis and/or treatment make a difference to prognosis and/or life expectancy? If so, is there is an optimum period of time within which a person should receive treatment? Has this differed over time?
Many cohorts have shown that improving life expectancy depends on the time point of starting combination ART. Initial studies after introduction of potent ART demonstrated that patients with presumed transmission via injecting drug use had lower life expectancies than did those from other transmission groups.127 Life expectancy was lower in patients with lower baseline CD4 cell counts than in those with higher baseline counts, in particular for CD4 cell counts below 100 cells. Moreover, normalisation of T4/T8 ratio appears mostly only achievable upon early ART initiation and has been associated with a lower risk for non-AIDS-defining events.128
(b) Is the prognosis or life expectancy different for a person who is co-infected with HCV and/or HBV compared to a person infected solely with HIV?
The life expectancy for patients infected with HIV and additional hepatitis B and/or C coinfection is different due to the increased risk of dying from concomitant liver disease. Indeed, within the EuroSIDA cohort, patients with hepatitis C antibodies had a nine-fold higher risk of dying from liver disease which was even higher in the presence of detectable hepatitis C replication.129 Similarly, data from the UK demonstrated increased rates of all-cause and liver-related mortality among individuals with hepatitis B or C co infection as well as HIV infection, highlighting the need for primary prevention and access to effective hepatitis treatment for HIV-positive individuals.130 Neither hepatitis B nor hepatitis C coinfection were associated with increased AIDS-related mortality.
Because of the increased risk of dying from liver disease in the setting of chronic hepatitis coinfection, in particular with progredient immunodeficiency,131 treatment of underlying hepatitis B and C is of utmost importance. Whereas early in the HIV epidemic, there were no satisfactory treatment options for HBV or HCV, this has fortunately dramatically changed over time (see also Expert Report to the Infected Blood Inquiry: Hepatitis).
Although successful antiviral combination therapy for HIV and subsequent immune reconstitution can slow down the faster fibrosis progression in HCV coinfection, risk of hepatic decompensation still remains higher in ART-treated HIV/HCV coinfected individuals versus HCV-monoinfected subjects.132 Sustained virological response (SVR) or cure after interferon (IFN) and ribavirin (RBV) combination therapy tended to be significantly lower in HIV-coinfected individuals particularly in genotype 1 patients. Therefore, the EACS guidelines recommended longer treatment durations of up to 72 weeks for genotype 1 and 4 patients who still had a detectable HCV-RNA four weeks after starting HCV therapy and at least a >2 log drop in HCV-RNA at week 12 of HCV therapy.133 Treatment for genotype 2 and 3 could last up to 48 weeks. Considering the longer HCV treatment durations, a higher risk for cumulative interferon and ribavirin related toxicity existed for HIV coinfected individuals. In the era of all oral direct acting anti-viral combination therapy, similar SVR rates have been observed for HIV-coinfected and HCV-monoinfected subjects.134 For HBV coinfection, a tenofovir-containing ART has become the standard of care with similar good outcomes as in treatment of HBV mono-infection. Hepatitis delta superinfection in patients with HIV/HBV coinfection remains a tremendous challenge as liver fibrosis progression is fastest and risk of dying from liver disease is particularly high. Unfortunately, treatment options are still poor in hepatitis D and usually consist of interferon-based strategies.
To what extent, and how, does HIV affect people with:
(a) haemophilia, (b) von Willebrand disease, (c) thalassaemia and (d) sickle cell anaemia differently from those who do not have a bleeding or blood disorder?
The impact of HIV on people with haemophilia, their families and the services providing specialist care has been profound and multifactorial.135, 136 This response focusses specifically on the clinical issues.
Overall, the clinical course of HIV and AIDS is not greatly affected by transmission risk group. The US National Cancer Institute evaluated the age-specific relative risk of progression from HIV seroconversion to onset of AIDS in people with haemophilia and homosexual men.101 Prospective follow-up data from HIV seroconversion to AIDS was analyzed for people with haemophilia in the Multicenter Hemophilia Cohort Study and for homosexual men in the International Registry of Seroconverters. Follow-up was censored at 1 July 1987 to obtain natural history estimates unaffected by therapies that were widely used after this date. The age-specific relative hazard of progression was estimated using nonparametric proportional hazards models and the baseline hazard function was described using spline models. Among the 373 children and adults with haemophilia and the 1020 adult homosexual men, each 10-year increment in age at seroconversion was associated with a 1.6- and 1.4-fold increase in the hazard of progression, respectively.101 The effect of age was highly significant among people with haemophilia. The magnitude of the effect was consistent in different cohorts of homosexual men, although it was not nominally significant. Furthermore, there was a significant increase (1.9-fold higher) in progression rates among homosexual men above rather than below 35 years of age at seroconversion. After adjusting for age, progression rates among people with haemophilia were significantly slower, possibly because Kaposi's sarcoma was rare.
A Canadian analysis of causes of death in people with haemophilia between 1980 and 1995 showed that HIV attributable deaths (including co-infections) accounted for the majority (80-90%) in HIV positive patients. In contrast death due to haemorrhage was higher in both HIV positive and HIV negative patients.137 Unlike non-haemophilia patients where cause of death was mainly opportunistic infection, cause of death in haemophilia patients was mainly intracranial haemorrhages and cirrhosis of liver.
Data from the UK showed that age at the time HIV seroconversion had a significant influence on survival rates amongst people with haemophilia, with longer survival seen amongst those who seroconverted at younger ages. The age-gradient in survival was not explained by deaths expected in the absence of HIV infection or by confounding with other factors such as haemophilia type or severity.138
An Italian study conducted a survival analysis among the cohort of HIV-positive people with haemophilia with AIDS at the Italian Haemophilia Registry. This study also showed that younger age at HIV seroconversion and at AIDS diagnosis were associated with a longer survival.139 This study indicated an increasing survival from AIDS diagnosis to death over time, also as a result of the introduction of antiretroviral therapy. Survival trends were similar to those reported among homosexual men and intravenous drug users with AIDS, suggesting a similar access to the health-care system for individuals with AIDS.
In summary, the natural course of HIV/AIDS is similar with the exception of Kaposi sarcoma which is mostly found in men who have sex with men and is linked to coinfection with HHV8 infection (no KS cases have been observed in the Bonn Hemophilia cohort to date; n=420). In the Bonn cohort lowest survival rates were found for intravenous drug users most likely due to a higher risk for bacterial infections (endocarditis) and risk for overdose. The only HIV associated manifestation which may have an impact on an underlying bleeding disorder might be HIV-associated thrombopenia. However, bleeding complications in this context have been very rare.
In the setting of HIV and hepatitis coinfection the risk for liver toxicity under HIV therapy is enhanced. This would also be true for IVDU with high risk for hepatitis coinfection. Overall, no difference has been found regarding time point of antiretroviral treatment initiation or choice of drugs depending on transmission risk. There have been a few reports of increased bleeding episodes under commonly used HIV first generation protease inhibitors which, however, were not observed in the Bonn HIV-infected haemophilia cohort with a preventive high dose clotting factor substitution policy.140 Indeed, increased bleeding events have not been reported for any other HIV drug class.
There have been several case reports describing acquired F8 inhibitors in patients receiving interferon alpha for hepatitis C virus (HCV) treatment and in immune reconstitution inflammatory syndrome (IRIS) in patients being treated for human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS).141 While the potential impact of HCV or HIV drugs on triggering an inhibitor cannot be ruled out, the fact that this was only reported in case reports suggests a low incidence of these events. Therefore, in general there was no difference in treatment of HIV in individuals with a bleeding disorder with an inhibitor.
The UK Haemophilia Centre Doctors' Organisation (UKHCDO) manages the UK's national haemophilia database. The UKHCDO carried out a study on the impact of HIV on mortality rates of people with haemophilia between 1977 and 1999. Of 7,250 men with haemophilia, a majority of whom were also infected with hepatitis C, 1246 were HIV positive. During 1977-1984 annual mortality in severely haemophilic males was 0.9%. For those with HIV, annual mortality increased progressively from 1985 reaching over 10% during 1993-1996 before falling to 5% in 1997-1999, likely related to anti-retroviral treatment.142
Advice and information sharing in the context of HIV should be seen as an ongoing process rather than as a single event.
Information, explanations and advice being given to people diagnosed with or affected by HIV must be done with consideration of the complexity of the condition, existing treatments, transmission mechanisms and health literacy of the individuals concerned. Information may be given in a context where there is little detailed knowledge, pre-existing fear, and concerns about associated stigma and discrimination.143
Testing for HIV is increasingly commonplace and more often being done on an 'opt-out' basis as part of other investigations in which blood testing is undertaken, or as sexual health screening in a primary care setting.144 A reasonable knowledge base of any primary care clinician is expected in order to facilitate initial discussion with a patient who is found to be positive for HIV. A suitable environment with adequate time given for such discussion is required. It would be unusual to expect a primary care clinician to give more than an initial diagnosis and brief explanation. This is mindful of both the potential shock of the diagnosis, and that only a very small portion of the information discussed may be retained beyond the diagnosis.145
Most patients and families may consider looking online for further information, and signposting to authoritative NHS or third-sector resources should be considered and prepared.
Services that offer diagnostic testing for HIV should have an agreed pathway into specialist HIV care for people who are diagnosed HIV positive. People who have a new diagnosis of HIV should expect to have their HIV status fully assessed by appropriately trained staff within two weeks of receiving an HIV-positive test result. Those with a new diagnosis of HIV who have symptoms and/or signs potentially attributable to HIV (including those of primary infection) should be referred for urgent (within 24 hours) specialist assessment. People who receive an HIV-positive diagnosis as a hospital inpatient should expect to be reviewed by appropriately trained staff within 24 hours if their admission may be HIV related.146
In a specialist HIV care setting, following full assessment, further details would be shared, and current level of understanding established, in a suitable, confidential environment. Adequate time for extensive, ongoing discussion over a number of clinic visits involving appropriate members of the multidisciplinary team should be planned.
Following initial clinical assessment and baseline investigations, a person with HIV should expect to have information about what HIV is and how it can affect their health and wellbeing. The person should be given information of the current stage of their HIV infection, including the CD4 count and viral load, any associated complications, their options for starting antiretroviral treatment and other associated interventions. This information should include choices of suitable drug combinations with risks and benefits. There should be clear understanding that HIV therapy is a treatment and not a cure, and requires long-term commitment and high levels of adherence. The arrangements for follow-up and monitoring should be outlined.
Information on prognosis will depend on the stage of infection. However, for the majority of people in the UK, newly diagnosed, asymptomatic and able to access treatment, life expectancy approximates to that of the general population. Information should be provided on how to maintain optimum health, including advice on smoking cessation, recreational drug use, diet and exercise.
The implications of the diagnosis for others, particularly sexual partners and children, need to be explored with the patient thoroughly and sensitively.
How HIV can and cannot be transmitted to others should be discussed and reassurance provided that HIV is not transmitted via casual contact. The impact of effective treatment preventing sexual and vertical transmission should be explained.
Disclosure to others and support available from practitioners with skills in partner notification should be explored. This should be provided via sexual health services or within the HIV service by those with sufficient expertise.
People living with HIV should be offered written and verbal information, which is culturally and age appropriate, about prevention of HIV transmission, including mechanisms for partners to access pre- and post-exposure prophylaxis (PrEP and PEP) if relevant.
People living with HIV should be aware of the importance of avoiding future sexually transmitted infections to which they may be more susceptible and, if acquired, may make them more infectious to others in the context of sexual transmission of HIV.
People who have been diagnosed HIV positive should be made aware of the legal position in relation to HIV transmission and how to protect themselves from prosecution. This should occur (and be documented) at the time of their initial diagnosis and subsequently as indicated.
Desires and plans for parenthood should be discussed with all people living with HIV during initial assessment and revisited periodically during ongoing care and management. Women with HIV should be reassured that pregnancy will not adversely affect the progression of their HIV infection.
People with HIV should be aware that, with effective treatment and life expectancy near-equivalent to that of the general population, they can expect their children to reach adulthood and to become a grandparent.
People with HIV should be aware of the risks of vertical transmission of HIV to their baby (mother-to-child). For women who are on effective therapy and who avoid breastfeeding, the risk of vertical transmission is less than 1:1000. People with HIV should expect to have accurate information about their own risks of vertical transmission during pregnancy, childbirth and breastfeeding, and how to minimise these risks. This will include the management of their pregnancy147 and birth to reduce the risk of infection for the baby. The baby will need to be tested for HIV at birth and at regular intervals for up to two years. All patients benefit from pre-conceptual advice with regard to lifestyle choices and general health. This is particularly so if there is a pre-existing illness.
People with HIV should expect to have information about safe conception within a sero-different relationship, including pre-conception advice for themselves and their partners. People with an undetectable viral load cannot pass HIV to their sexual partners. The risk of vertical transmission rises if an HIV-negative woman acquires HIV during pregnancy or whilst breast feeding when viral load would be high. Information should be given about PrEP and PEP in this situation.
Women with HIV should expect to have information on how to feed their baby. In the UK, women with HIV are advised to avoid breastfeeding and to use replacement feeding. Women who elect to breastfeed their babies should be given specialist support and advice on how to manage this and the indications to stop breastfeeding.
People living with HIV should have access to accurate information and support for pregnancy choices, including contraception and abortion. Information should be shared on the potential for interactions between oral contraceptives and ART to avoid contraception or ART failure.
People living with HIV who require investigation and treatment for infertility should be given information on ways to access fertility/conception services.
HIV, hepatitis B and hepatitis C are blood-borne viruses and patients who have ever tested positive for these may not donate blood irrespective of whether they have been symptomatic or not.148 All donated blood is routinely tested for hepatitis B, C and E as well as HIV.149
Implications of the diagnosis for family members, particularly for children and sexual partners, need first to be explored with the patient thoroughly and sensitively: working out with the patient to clarify who does and who does not need to be aware of the diagnosis, and then supporting the patient with sharing the information.
For family members who are aware of the diagnosis, an explanation of how HIV can and cannot be transmitted to others should be discussed and reassurance provided that HIV is not transmitted via casual contact. The impact of effective treatment on the longevity and wellbeing of people with HIV and its ability to prevent sexual and vertical transmission should be explained. Signposting to other reliable sources of information and support about HIV should be given (for example, NAM Aidsmap, Terence Higgins Trust, National Aids Trust).
All newly diagnosed HIV-positive patients attending adult HIV services should have any children identified, tested – or testing history obtained (and evidenced) – and the information clearly documented. The risks of vertical transmission should be assessed and discussed. This should be handled sensitively and supportively with a partnership approach, bearing in mind that the health of the child/children, who may have acquired HIV, is a priority.
All children (defined as those who have not yet reached their 18th birthday) born to people living with HIV should be assessed for risks of vertical transmission. Testing children at risk of HIV acquisition should be discussed with at least one of the child's parents or legal guardians, and appropriate testing and follow-up organised with paediatric colleagues.146
Sexual partners of people who test positive should receive a prompt offer and recommendation of an HIV test through partner notification procedures.
The clinician-patient relationship is important at any time but particularly for patients with long-term conditions that have significant impact on the patient, their family, their relationships and their social world. People with HIV require professional care and peer support from trusted multidisciplinary teams to maintain support and continuity. People should be assured of not just care but understanding, respect and excellence.
The GP and primary care team are likely to be involved with the initial presentation and diagnosis of illness, onward referral and support with the psychological and social aspects of care for the patient and their families. Throughout the course of the illness, this may include provision of information orally but may also include appropriate written material or signposting to relevant trusted websites or specific patient support groups and networks. It will include the management of test results, medication and referrals to community groups and secondary care. Everyone living with HIV should have access to appropriate peer support, ideally embedded within the clinical service and an integral part of the multidisciplinary team. Different learning and health literacy needs should be catered for, including the use of pictorial explanation and support and encouragement of patient self-recording,150 which can increase understanding and retention of information.
Signposting and providing written information for a patient to consider after a more in-depth secondary care review is important given information retention issues, which are exacerbated if the patient is anxious during a consultation.
People with HIV should know how information about their diagnosis is shared among healthcare professionals and be part of that decision. Sharing of information can cause anxiety and concern but is often critical for safe prescribing given the common interactions with drugs used to treat hepatitis151 and HIV.152 Of note, 91% of HIV patients, tested elsewhere, shared their HIV status with their registered GP.153
Specialist HIV care is based on a multidisciplinary team model and often arranged in clinical networks. This means that a number of people and teams may be involved. While each may have specific roles in the management of the patient's care and treatment, they are also likely to have shared roles and responsibilities. Good communication between everyone involved is essential for best outcomes and if the patient is to have confidence in their care.
Living with HIV requires lifelong adjustments and management. With the drive towards personalised medicine and shared decision making, patients should be aware of the natural course of their condition, enabling them to seek advice and help when appropriate and to manage the impact of HIV and its complications on their daily lives. As with other long-term health conditions, people living with HIV should optimise self-management and access peer-support opportunities to promote their physical and mental health and overall well-being. Self-management approaches help people living with HIV gain confidence, skills and knowledge to manage their own health resulting in improvements in quality of life and independence.
Clinicians should make no assumptions about patient preference to treatment and care options in HIV. Both Realistic Medicine154 and NHS England155 highlight the importance of effective shared decision making to achieve optimal patient care. It is important to listen to patient preferences and share decision making having ensured that the patient has the information needed to make an informed choice in an equal partnership.
BHIVA care standards recommend that people living with HIV should be actively involved in decisions about their own health and social care, and that people seeking care for HIV are participants in, not just beneficiaries of, health systems.146 There should be active involvement of people who use services in the design, planning, delivery and review of those services. Active engagement in decision making may require support and resources for both people living with HIV and service providers. Service providers should be able to demonstrate their commitment to participation through identification of strategies, pathways and resources for both individual engagement in decision making and community engagement in service provision.
HIV antiretroviral therapy (ART), when taken daily, is able to stop the replication of HIV (with undetectable viral load in the blood of an HIV positive patient). Patients with undetectable virus do not transmit HIV to others and, in general, their immune system recovers over time. However, if treatment is stopped, detectable virus will return, usually within weeks. One of the great challenges for research is how to enable patients to stop therapy and remain with undetectable virus, free of the complications of infection. Cure may be 'sterilising', where there is no virus left in the body, or 'functional' where virus remains in the body but an individual does not require treatment.
The main challenges to developing a cure of HIV are as follows: (i) The virus is able to integrate itself into the DNA of infected cells. This is often referred to as 'latent' virus. This viral DNA remains as a reservoir, even if treatment is able to prevent the creation of new virus, and can re-emerge when treatment is stopped. (ii) These latently infected cells can expand in number even when a patient is on effective treatment. (iii) Although treatment may mean viral replication is not detectable in blood using standard tests, there can often be low-level production of virus which maintains the infection. (iv) Reservoirs of infected cells are found in the cells of the immune system throughout the body, particularly in the tissue around the intestines, and are not therefore amenable to being removed. (v) Current HIV treatment, although lifelong, is generally well tolerated by most patients so new treatments need to be both highly effective and safe to be considered for widespread use.156, 157
The most high-profile approach to cure, with widespread media coverage, is bone marrow transplantation (BMT). The process of BMT involves using potent chemotherapy and irradiation to ablate an individual's immune system, removing the sites of viral reservoirs. Stem cells from a different patient 'allogenic' are then infused to restore the immune system. In the setting of HIV infection, stem cells are chosen from a donor with a particular naturally occurring genetic type, known as 'CCR5 delta32 deletion homozygotes'. This genetic type is relatively rare. Individuals who carry two copies of the detla32 deletion are resistant to HIV infection.
BMT using this approach has been able to cure only a very small number of patients. It is not suitable for widespread used in HIV cure, primarily because the risks of the procedure are high (particularly related to toxicity of drugs and vulnerability to infection) and cannot be justified for patients who are healthy on their medication. In addition, individuals with the appropriate genotype are relatively uncommon in the population (approximately 1%). The procedure has thus far only been used for six individuals who have a life-threatening condition (other than HIV) that require the procedure. To date, two/three have achieved what is thought to be a sterilizing cure.64
There is ongoing research to develop milder forms of the procedures that could be suitable for more widespread use. BMT using a patient's own cells requires less intensive chemotherapy and there are studies ongoing exploring the potential for using gene editing techniques to stop CCR5 gene expression in an infected individual's cells. Tools such as zinc-finger nucleases and CRISPR/Cas9 editing offer promise, but are not yet sufficiently well developed to offer routinely.
Progress in the development of an HIV vaccine that can prevent infection has been disappointing to date, but the approaches taken to enhance the body's specific immune response to HIV are being explored in HIV cure therapy. In combination with other agents (see below), there have been clinical studies suggesting that a vaccine producing strong T cell responses may help control virus, though falling short of functional cure. Ongoing work is focused on developing stronger immune responses that can recognise the broad range of viral targets present during a natural infection.
Clinical studies have started investigating antibodies that have been selected to neutralise a wide range of viral strains. When given as an infusion, they may help clear free virus from a patient's blood and potentially clear HIV-infected cells. The initial hope is that periodic infusion of antibodies may be able to control HIV sufficiently for patients to stop their daily HIV treatment, although it is likely that combinations of antibodies will be needed to prevent viral resistance. Work is ongoing to engineer new antibody-like molecules that can target a wider range of viral targets.
Treatment with cell-based therapies have transformed cancer care in recent years and are being evaluated for a role in HIV cure. Known as 'CAR-T therapy' ('chimeric antigen receptor – T cell'), T cells from a person's own immune system are removed and modified to include molecules that target a specific cell type (for example, lymphoma or HIV infection), before being given back to patients. In the case of HIV, the molecules included may be based on fragments of broadly neutralising antibodies (bNAbs, see above) specific for HIV. Other cell types (for example, iNKT cells) are being investigated that might allow 'off the shelf' treatments. Work in the area of cellular therapy for HIV cure is in its early stages.
The approach of 'kick-and-kill' is exploring the likely scenario that successful cure based on strengthening the immune system's response to HIV-infected cells is likely to first require activation of latently infected cells so they can be recognised. A range of drugs (known as 'latency reversal agents' [LRAs]) is being studied including HDAC inhibitors (for example, romidespin, vorinostat), toll-like receptor agonists (for example, vesatolimod) and disulfiram. The first randomised trial for HIV cure therapy, RIVER, was completed in 2019, but found no evidence of reduction in reservoirs of HIV from a combination of LRA (vorinostat) and therapeutic vaccination.158
Inflammation as a result of HIV infection is associated with a range of health impacts including cardiovascular disease and malignancy. Drugs that reduce inflammation and weaken immune responses (for example, sirolimus) have been associated with reducing HIV reservoirs in small studies and are being actively investigated.
The understanding of how HIV causes long-term infection continues to deepen, and in doing so provides new opportunities for potential cure. However, none of the above approaches has shown sufficient promise to be available for widespread clinical use in the next 10 years. A combination of approaches is likely to be needed to effect a cure. With over 80% of those infected with HIV living in sub-Saharan Africa, most healthcare systems will have insufficient resources to deliver a complex medical intervention at scale. Consideration needs to be given to prioritising therapies that can be delivered in resource-limited settings.
To be answered in the Supplementary Report (see addendum)
To be answered in the Supplementary Report (see addendum)
To be answered in the Supplementary Report (see addendum)
The general principle of infection control when treating all patients including those with HIV is one of 'standard precautions' for all patient care. Standard precautions are the minimum infection prevention practices that apply in any setting where healthcare is delivered, regardless of suspected or confirmed infection status of the patient. Standard precautions are designed to reduce the risk of transmission of blood-borne and other pathogens to healthcare workers and other patients from both recognised and unrecognised sources.
Standard precautions include the following
If admitted to hospital, HIV-positive patients do not need to be isolated in a single room unless they are suspected of having another condition which may be transmissible to others via the airborne route – for example, tuberculosis.
There are currently no vaccines available against HIV, although policies and procedures for the reporting of needlestick and splash injuries will ensure that a risk assessment takes place and, if HCWs are exposed to HIV post-exposure, anti-viral prophylaxis will be provided.
The Health and Safety Executive (HSE) provides guidance for procedures for taking blood specimens and for clinical laboratories handling samples from patients with HIV in the course of their treatment.160 This includes the use of standard precautions for all patients, as all blood samples should be considered potentially infectious. Therefore, gloves should be worn when taking blood for all patients. 'Danger of infection' labels should be used for samples known to pose a risk to staff handling the sample.
In 1972, the Rosenheim Advisory Group issued good practice guidelines to prevent the transmission of hepatitis B in renal dialysis and transplant units. Since then, with the identification of both HIV and HCV, new guidance 'Good practice guidelines for renal dialysis/transplantation units and the prevention of and control of blood-borne virus (BBV) infection', was published in 2002 and remains current.161 Patients with HBV should ideally be dialysed in separate isolation facilities. Where these are not currently available, patients should be segregated in a separate area from other patients during dialysis. Patients with HCV should also be segregated from uninfected patients during dialysis. Segregation of patients with HIV should be considered, based on a local risk assessment. Because of the risk of cross-infection, patients with different BBV infections should not be dialysed in a single segregated area at the same time.
Separate dedicated dialysis machines should be used for patients with HBV. Dedicated machines are not required for patients with HCV or HIV provided that cleaning and disinfection processes are properly carried out between patients according to the manufacturers' instructions. Whenever possible, staff should nurse only infected or uninfected patients during a shift. If this is not practicable, more experienced staff should be assigned the task of caring for a mixed group of patients.
Whenever blood or body fluid is present, there is a potential risk of blood-borne transmission and appropriate protective measures should be taken. Only leakage of blood or body fluids produces a risk of BBV infection, and simple hygiene measures are adequate to prevent transmission. However, whilst hygienic preparation is acceptable, current HSE guidance stipulates that bodies with BBV infections can only be embalmed if additional transmission-based precautions are in place with appropriate robust measures for the use of sharps (for example, minimise use or use safer sharps devices) as this presents significant risk of exposure to workers.162
Centres providing facilities for the processing and storage of gametes and embryos for use in fertility treatments must carry out testing for hepatitis B, hepatitis C and HIV, and devise a system for storage which clearly separates gametes and embryos from individuals who have tested positive from those who have tested negative.163
To be answered in the Supplementary Report
To be answered in the Supplementary Report
References
1 United Nations AIDS, 2015, Terminology Guidelines, accessed 28 January
2020, available at <https://www.unaids.org/sites/default/files/media_asset/2015_terminology_guidelines_en.pdf>.>.
2 Sharp PM, Hahn BH, 2011, 'Origins of HIV and the AIDS Pandemic', Cold Spring Harbor Perspectives in Medicine, 1(1), a006841.
3 Coffin JM, 1995, 'HIV Population Dynamics in Vivo: Implications for Genetic Variation, Pathogenesis, and Therapy', Science, 267(5197), 483-489.
4 Barré-Sinoussi F, Chermann JC, Rey F et al., 1983, 'Isolation of a T-lymphotropic Retrovirus from a Patient at Risk for Acquired Immune Deficiency Syndrome (AIDS)', Science, 220(4599), 868-871.
5 Gallo RC, Salahuddin SZ, Popovic M, et al., 1984, 'Frequent Detection and Isolation of Cytopathic Retroviruses (HTLV-III) from Patients with AIDS and at Risk for AIDS', Science, 224(4648), 500-503.
6 Coffin J, Haas A, Levy JA, et al., 1986, 'What to Call the AIDS Virus?', Nature, 321(6065), 10.
7 Morbidity and Mortality Weekly, 1981, 'Epidemiologic Notes and Reports, Pneumocystis Pneumonia—Los Angeles', Morbidity and Mortality Weekly Report, 30(21), 1-3.
8 Centers for Disease Control ,1982, 'A Cluster of Kaposi's Sarcoma and Pneumocystis Carinii Pneumonia among Homosexual Male Residents of Los Angeles and Orange Counties, California', Morbidity and Mortality Weekly Report, 31(23), 305-307.
9 Centers for Disease Control and Prevention, 1982, 'Current Trends Update on Acquired Immune Deficiency Syndrome (AIDS) – United States', Morbidity and Mortality Weekly Report, 31(37), 507-508.
10 World Health Organisation, 2007, WHO Case Definitions of HIV for Surveillance and Revised Clinical Staging and Immunologic Classification of HIV-related Disease in Adults and Children, World Health Organization, Geneva, accessed 28 January 2020, available at <http://www.who.int/hiv/pub/guidelines/HIVstaging150307.pdf>.>.
11 Centers for Disease Control, 1992, '1993 Revised Classification System for HIV Infection and Expanded Surveillance Case Definition for AIDS Among Adolescents and Adults' Morbidity and Mortality Weekly Report, 41(RR-17), 1-19.
12 Centers for Disease Control, 2014, 'Revised Surveillance Case Definition for HIV Infection–United States, 2014' Morbidity and Mortality Weekly Report, 63(RR-03), 1-10, accessed 28 January 2020, available at <https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6303a1.htm>.>.
13 Centers for Disease Control and Prevention, 2019, Terms, Definitions, and Calculations Used in CDC HIV Surveillance Publications, accessed 28 January 2020, available at <https://www.cdc.gov/hiv/statistics/surveillance/terms.html>.>.
14 Centers for Disease Control, 1981, 'Kaposi's Sarcoma and Pneumocystis Pneumonia Among Homosexual Men–New York City and California', Morbidity and Mortality Weekly Report, 30(25), 305-307.
15 Du Bois RM, Branthwaite MA, Mikhail JR, Batten JC, 1981, 'Primary Pneumocystis Carinii and Cytomegalovirus Infections', The Lancet, 2(8259), 1339.
16 Terrence Higgins Trust, Our History: How it all began, accessed 28 January 2020, available at <https://www.tht.org.uk/our-work/about-our-charity/our-history>.>.
17 Gallo RC, Sarin PS, Gelmann EP, et al., 1983, 'Isolation of Human T-cell Leukemia Virus in Acquired Immune Deficiency Syndrome (AIDS)', Science, 220(4599), 865-867.
18 Altman L, 2008, Discoverers of AIDS and Cancer Viruses Win Nobel, New York Times, 7 October, accessed 28 January 2020, available at <https://www.nytimes.com/2008/10/07/health/07nobel.html>.>.
19 Cohen J, 1997, 'The Rise and Fall of Project SIDA', Science, 278(5343), 1565-1568.
20 IAS, Conferences Timeline, accessed 28 January 2020, available at <https://www.iasociety.org/Conferences/Previous-Conferences/Timeline>.
21 Serwadda D, Sewankambo NK, Carswell JW, et al., 1985, 'Slim Disease: a New Disease in Uganda and its Association with HTLV-III Infection', The Lancet, 326(8460), 849-852.
22 Centers for Disease Control, 1985, 'Testing Donors of Organs, Tissues, and Semen for Antibody to Human T-lymphotropic Virus Type III/Lymphadenopathy-associated Virus', Morbidity and Mortality Weekly Report, 34(20), 294.
23 Alexander TS, 2016, 'Human Immunodeficiency Virus Diagnostic Testing: 30 Years of Evolution', Clinical and Vaccine Immunology, 23(4), 249-253.
24 Brettle RP, Bisset K, Burns S, et al., 1987, 'Human Immunodeficiency Virus and Drug Misuse: the Edinburgh Experience', British Medical Journal (Clinical Research Ed.), 295(6595), 421-424.
25 Expert Advisory Group on AIDS, Expert Advisory Group on AIDS, accessed 28 January 2020, available at <https://www.gov.uk/government/groups/expert-advisory-group-on-aids>. >.
26 House of Lords, 2011, Select Committee on HIV and AIDS in the United Kingdom, 1st Report of Session 2010-2012, No Vaccine, No Cure: HIV and AIDS in the United Kingdom, HL Paper 188, London, The Stationery Office Limited, accessed 28 January 2020, available at <https://publications.parliament.uk/pa/ld201012/ldselect/ldaids/188/188.pdf>.>.
27 National AIDS Trust, What We Do – Our History, accessed 28 January 2020, available at <https://www.nat.org.uk/what-do-we-do/nat/our-history>. >.
28 BBC, 2017, How Princess Diana Changed Attitudes to AIDS, 5th April, accessed 28 January 2020, available at <https://www.bbc.co.uk/news/av/magazine-39490507/how-princess-diana-changed-attitudes-to-aids>.>.
29 Burgess A, 2017, 'The Development of Risk Politics in the UK: Thatcher's "Remarkable" but Forgotten "Don't Die of Ignorance" AIDS Campaign', Health, Risk & Society, 19:5-6, 227-245.
30 Schild GC, Cope JE, 1989, 'Medical Research Council AIDS Directed Program: Program Plan and Research Opportunities', Journal of Acquired Immune Deficiency Syndrome, 2(6), 595-602.
31 Stimson GV, 1995, 'AIDS and Injecting Drug Use in the United Kingdom, 1987-1993: The Policy Response and the Prevention of the Epidemic', Social Science & Medicine, 41(5), 699-716.
32 Fischl MA, Richman DD, Grieco MH et al., 1987, 'The Efficacy Azidothymidine (AZT) in the Treatment of Patients with AIDS and AIDS Related Complex. A Double Blind Placebo Controlled Trial', New England Journal of Medicine, 317(4), 185-191.
33 Stimson GV, Alldritt L, Dolan K, Donoghoe M, 1988, 'Syringe Exchange Schemes for Drug Users in England and Scotland', British Medical Journal (Clinical Research Edition, 296(6638), 1717-1719.
34 Glanz A, Byrne C, Jackson P, 1989, 'Role of Community Pharmacies in Prevention of AIDS Among Injecting Drug Misusers: Findings of a Survey in England and Wales', British Medical Journal, 299(6707), 1076-1079.
35 Gill ON, Adler MW, Day NE, 1989, 'Monitoring the Prevalence of HIV', British Medical Journal, 299(6711), 1295–1298.
36 Wadsworth J, Field J, Johnson AM, et al., 1993, 'Methodology of the National Survey of Sexual Attitudes and Lifestyles', Journal of the Royal Statistical Society, 156(3), 407-421.
37 Nicolson DJ, 2003, 'Television Coverage of AIDS and Transmission of the AIDS Virus', Sexually Transmitted Infections, 79(2), 170.
38 BBC, 1991, 1991: Giant of rock dies, 24 November, accessed 28 January
2020, available at <http://news.bbc.co.uk/onthisday/hi/dates/stories/november/24/newsid_2546000/2546945.stm>. >.
39 Goldsmith MF, 1998, 'Dec 1 Designated World AIDS Day: Message is "Join the Worldwide Effort"', JAMA, 260(20), 2969-2970.
40 United Kingdom Advisory Panel for Healthcare Workers Infected with Bloodborne Viruses, 2004, Annual Report: 1st April 2003 to 31st March 2004, accessed 28 January 2020, available at <https://webarchive.nationalarchives.gov.uk/20140712041319/http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/BloodborneVirusesAndOccupationalExposure/UKAP/>. >.
41 Department of Health, 1992, The Health of the Nation – A Strategy for Health in England, London, White Paper, Her Majesty's Stationery Office.
42 Gabbay J, 1992, 'The Health of the Nation', British Medical Journal, 305(6846), 129-130.
43 Select Committee on HIV and AIDS in the United Kingdom, 2011, Memorandum by the Medical Research Council (HAUK 58), in HIV and AIDS in the United Kingdom. Written Evidence, London, House of Lords, accessed 28 January 2020, available at <https://www.parliament.uk/documents/lords-committees/hivaids/WrittenEvAtoZHIVAIDS.pdf>.>.
44 Taylor A, Goldberg D, Emslie J, et al., 1995, 'Outbreak of HIV Infection in a Scottish Prison', British Medical Journal, 310(6975), 289–292.
45 Oleske J, Bardequez A, 1994, 'Research Shows AZT Therapy Reduces Mother-Child AIDS Transmission', New Jersey Medicine, 91(4), 274.
46 Public Health England, 2019, UK National HIV Surveillance Data Tables, accessed 28 January 2020, available at <https://www.gov.uk/government/statistics/hiv-annual-data-tables>.>.
47 British HIV Association, BHIVA: About Us, accessed 28 January 2020, available at <https://www.bhiva.org/AboutBHIVA>.>.
48 Perelson AS, Neumann AU, Markowitz M, et al., 1996, 'HIV-1 Dynamics in Vivo: Virion Clearance Rate, Infected Cell Life-Span, and Viral Generation Time', Science, 271(5255), 1582-1586.
49 Hammer SM, Squires KE, Hughes MD, et al., 1997, 'A Controlled Trial of Two Nucleoside Analogues plus Indinavir in Persons with Human Immunodeficiency Virus Infection and CD4 Cell Counts of 200 per Cubic Millimeter or Less. AIDS Clinical Trials Group 320 Study Team', New England Journal of Medicine, 337(11), 725-733.
50 British HIV Association, 1997, 'British HIV Association Guidelines for Antiretroviral Treatment of HIV Seropositive Individuals. BHIVA Guidelines Co-ordinating Committee', The Lancet, 349(9058), 1086-1092.
51 Rogers PA, Sinka KJ, Molesworth AM, et al., 2000, 'Survival After Diagnosis of AIDS Among Adults Resident in the United Kingdom in the Era of Multiple Therapies', Communicable Disease and Public Health, 3(3), 188-194.
52 Croxford S, Kitching A, Desai S, et al., 2017, 'Mortality and Causes of Death in People Diagnosed with HIV in the Era of Highly Active Antiretroviral Therapy Compared with the General Population: An Analysis of a National Observational Cohort', The Lancet Public Health, 2(1), e35-e46.
53 Department of Health, 1999, Reducing Mother to Baby Transmission of HIV, London, HSC 1999/183.
54 United Nations General Assembly, 2000, United Nations Millennium Declaration, A/RES/55/2, accessed 28 January 2020, available at <https://www.un.org/en/development/desa/population/migration/generalassembly/docs/globalcompact/A_RES_55_2.pdf>. >.
55 The Global Fund, 2019, Global Fund Overview, 28 June, accessed 28 January 2020, available at <https://www.theglobalfund.org/en/overview/>. >.
56 Adler MW, French P, McNab A, et al., 2002, 'The National Strategy for Sexual Health and HIV: Implications for Genitourinary Medicine', Sexually Transmitted Infections, 78(2), 83-86.
57 Bird SM, Brown A JL, 2001, 'Criminalisation of HIV Transmission: Implications for Public Health in Scotland', British Medical Journal, 323(7322), 1174-1177.
58 Stramer SL, Glynn SA, Kleinman SH, et al., 2004, 'National Heart, Lung, and Blood Institute Nucleic Acid Test Study Group. Detection of HIV-1 and HCV Infections Among Antibody-negative Blood Donors by Nucleic Acid-amplification Testing', New England Journal of Medicine, 351(8), 760-768.
59 McDaid LM, Hart GJ, 2011, 'Increased HIV Testing and Reduced Undiagnosed Infection Among Gay Men in Scotland, 2005-8: Support for the Opt-out Testing Policy?', Sexually Transmitted Infections, 87(3), 221–224.
60 World Health Organisation, 2003, Treating 3 Million by 2005: Making it happen: The WHO Strategy: The WHO and UNAIDS Global Initiative to Provide Antiretroviral Therapy to 3 Million People with HIV/AIDS in Developing Countries by the End of 2005, World Health Organization, Geneva.
61 HIV.GOV, 2018, PEPFAR, What is PEPFAR?, 28 November, accessed 28 January 2020, available at <https://www.hiv.gov/federal-response/pepfar-global-aids/pepfar>. >.
62 Disability Discrimination Act 2005.
63 Department of Health, 2007, Health Clearance for Tuberculosis, Hepatitis B, Hepatitis C and HIV: New Healthcare Workers, London, Department of Health, accessed 28 January 2020, available at <https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/382152/health_clearance_tuberculosis_hepatitis_hiv.pdf>. >.
64 Hütter G, Nowak D, Mossner M, et al., 2009, 'Long-term Control of HIV by CCR5 Delta32/Delta32 Stem-cell Transplantation', New England Journal of Medicine, 360(7), 692-698.
65 Equality Act 2010.
66 Grant RM, Lama JR, Anderson PL, et al., 2010, 'Pre-exposure Chemoprophylaxis for HIV Prevention in Men who Have Sex with Men', New England Journal of Medicine, 363(27), 2587-2599.
67 Cohen MS, Chen YQ, McCauley M, et al., 2011, 'Prevention of HIV-1 Infection with Early Antiretroviral Therapy', New England Journal of Medicine, 365(6), 493-505.
68 British HIV Association, HIV Testing Week 2012, accessed 28 January 2020, available at <https://www.bhiva.org/HIV-Testing-Week-2012>.>.
69 Pebody R, 2012, From Today, HIV Treatment is Free for All who Need it in England, 1 October, NAM aidsmap, accessed 28 January 2020, available at <https://www.aidsmap.com/news/oct-2012/today-hiv-treatment-free-all-who-need-it-england>. >.
70 Williams I, Churchill D, Anderson J, et al., 2014, 'British HIV Association Guidelines for the Treatment of HIV-1-positive Adults with Antiretroviral Therapy 2012 (2013 Update)', HIV Medicine, 15(Suppl 1), 1–85.
71 United Nations AIDS, 90-90-90: Treatment for all, accessed 28 January 2020, available at <https://www.unaids.org/en/resources/909090>. >.
72 World Health Organisation, 2015, Guideline on When to Start Antiretroviral
Therapy and on Pre-exposure Prophylaxis for HIV, World Health Organization,
Geneva, accessed 28 January 2020, available at <https://apps.who.int/iris/bitstream/handle/10665/186275/9789241509565_eng.pdf;jsessionid=EA83F7B9D31A729727FF5323D4A828A9?sequence=1>.>.
73 British HIV Association, 2016, British HIV Association Guidelines for the Treatment of HIV-1-Positive Adults with Antiretroviral Therapy 2015 (2016 Interim update) London, British HIV Association, accessed 28 January 2020, available at <https://www.bhiva.org/file/RVYKzFwyxpgiI/treatment-guidelines-2016-interim-update.pdf>. >.
74 The Health and Social Care Act 2012.
75 McCormack S, Dunn D, 2015, 'Pragmatic Open-label Randomised Trial of Pre-exposure Prophylaxis: The PROUD Study', Conference Presentation on Retroviruses and Opportunistic Infections, Feb 23, 23-26.
76 National Aids Trust v National Health Service Commissioning Board (NHS England) [2016] EWHC 2005.
77 McCormack S, Dunn DT, Desai M, et al., 2016, 'Pre-exposure Prophylaxis to Prevent the Acquisition of HIV-1 Infection (PROUD): Effectiveness Results from the Pilot Phase of a Pragmatic Open-label Randomised Trial', The Lancet, 387(10013), 53-60.
78 Health Protection Scotland, 2019, Implementation of HIV PrEP in Scotland: First Year Report, Health Protection Scotland, accessed 28 January 2020, available at <https://www.hps.scot.nhs.uk/web-resources-container/implementation-of-hiv-prep-in-scotland-first-year-report/>. >.
79 Communicable Disease Surveillance Centre, 2018, Pre-exposure Prophylaxis for HIV (PrEP) Provision in Wales, Communicable Disease Surveillance Centre.
80 Delpech V, Desai M, 2017, 'Towards Elimination of HIV Amongst Gay and Bisexual Men in the United Kingdom, in 23rd Annual Conference of the British HIV Association, Liverpool, April 4.
81 Kirby T, 2018, 'The UK Reaches UNAIDS 90-90-90 Targets', The Lancet, 392(10163), 2427.
82 Nash S, Desai S, Croxford S, et al., 2018, Progress Towards Ending the HIV Epidemic in the United Kingdom: 2018 Report, Public Health England, accessed 28 January 2020, available at <https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/821273/Progress_towards_ending_the_HIV_epidemic_in_the_UK.pdf>. >.
83 Department of Health and Social Care, 2019, Health Secretary Announces Goal to end HIV Transmissions by 2030, Department of Health and Social Care, accessed 28 January 2020, available at <https://www.gov.uk/government/news/health-secretary-announces-goal-to-end-hiv-transmissions-by-2030>. >.
84 HIV Commission, Call for Written Evidence to Close on the 31st January 2020, accessed 28 January 2020, available at <https://www.hivcommission.org.uk/>. >.
85 Public Health England, 2019, Progress Towards Ending the HIV Epidemic in the United Kingdom 2018 Report − Summary, Key Messages and Recommendations, accessed 28 January 2020, available at <https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/813168/HIV_annual_report_2018_-_Summary_key_messages_and_recommendations.pdf>. >.
86 National Institute for Health and Care Excellence, 2016, HIV Testing: Increasing Uptake Among People Who May Have Undiagnosed HIV, NICE Guideline [NG60], accessed 28 January 2020, available at <https://www.nice.org.uk/guidance/ng60>.>.
87 British HIV Association, BHIVA/BASHH/BIA Adult HIV Testing Guidelines 2020, accessed 28 January 2020, available at <https://www.bhiva.org/file/5dfceab350819/HIV-Testing-Guidelines.pdf>.>.
88 Public Health England, 2016, Health Protection Report, Public Health England, 10(24), accessed 28 January 2020, available at <https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/541032/hrp2416.pdf>. >.
89 Joint United Kingdom (UK) Blood Transfusion and Tissue Transplantation Services Professional Advisory Committee, 2013, Guidelines for the Blood Transfusion Services in the UK, 8th edition, London, The Stationery Office.
90 Daar ES, Pilcher CD, Hecht FM, 2008, 'Clinical Presentation and Diagnosis of Primary HIV-1 Infection. Current Opinion in HIV and AIDS', Current Opinion HIV AIDS, 3(1), 10-15.
91 Fiebig EW, Wright DJ, Rawal BD, et al., 2003, 'Dynamics of HIV Viremia and Antibody Seroconversion in Plasma Donors: Implications for Diagnosis and Staging of Primary HIV Infection', AIDS, 17(13), 1871-1879.
92 Cooper D, Maclean P, Finlayson R, et al., 1985, 'Sydney AIDS Study Group. Acute AIDS Retrovirus Infection: Definition of a Clinical Illness Associated with Seroconversion', The Lancet, 325(8428), 537-540.
93 Kahn JO, Walker BD, 1998, 'Acute Human Immunodeficiency Virus Type 1 Infection', New England Journal of Medicine, 339(1), 33-39.
94 Hoenigl M, Green N, Camacho M, et al., 2016, 'Signs or Symptoms of Acute HIV Infection in a Cohort Undergoing Community-based Screening', Emerging infectious diseases, 22(3), 532.
95 Langford SE, Ananworanich J, Cooper DA, 2007, 'Predictors of Disease Progression in HIV Infection: A Review', AIDS Research and Therapy, 4(1), 11.
96 Levy JA, 2009, 'HIV Pathogenesis: 25 Years of Progress and Persistent Challenges', AIDS, 23(2), 147-160.
97 Gebara NY, El Kamari V, Rizk N, 2019, 'HIV-1 Elite Controllers: An Immunovirological Review and Clinical Perspectives', Journal of Virus Eradication, 5(3), 163.
98 Schwarze-Zander C, Blackard JT, Rockstroh JK, 2012, 'Role of GB Virus C in Modulating HIV Disease', Expert Review of Anti-Infective Therapy, 10(5), 563-572.
99 Safreed-Harmon K, Anderson J, Azzopardi-Muscat N, et al., 2019, 'Reorienting Health Systems to Care for People with HIV Beyond Viral Suppression', The Lancet, 6(12), HIV e869-e877.
100 O'Brien TR, Blattner WA, Waters D, et al., 1996, 'Serum HIV-1 RNA-levels and Time to Development of AIDS in the Multicenter Hemophilia Cohort Study', JAMA, 276(2), 105-110.
101 Rosenberg PS, Goedert JJ, Biggar RJ, 1994, 'Effect of Age at Seroconversion on the Natural AIDS Incubation Distribution. Multicenter Hemophilia Cohort Study and the International Registry of Seroconverters', AIDS, 8(6), 803-810.
102 National Institute for Health and Care Excellence, 2017, HIV Testing: Encouraging Uptake, Quality Standard [QS157], accessed 28 January 2020, available at <https://www.nice.org.uk/guidance/qs157/chapter/Quality-statement-3-HIV-indicator-conditions>. >.
103 British HIV Association, 2019, BHIVA Guidelines for the Routine Investigation and Monitoring of Adult HIV-1-Positive Individuals 2016 (2019 Interim Update), accessed 28 January 2020, available at <https://www.bhiva.org/monitoring-guidelines>. >.
104 Churchill D, Waters L, Ahmed N, et al., 2016, 'BHIVA Guidelines for the Treatment of HIV-1-Positive Adults with Antiretroviral Therapy 2015', HIV Medicine, 17(S4), s2-s104.
105 Rodger AJ, Cambiano V, Bruun T, et al., 2016, 'Sexual Activity Without Condoms and Risk of HIV Transmission in Serodifferent Couples When the HIV-Positive Partner Is Using Suppressive Antiretroviral Therapy', JAMA, 316(2), 171–181.
106 Rodger AJ, Cambiano V, Bruun T, et al., 2019, 'Risk of HIV Transmission Through Condomless Sex in Serodifferent Gay Couples with the HIV-Positive Partner Taking Suppressive Antiretroviral Therapy (PARTNER): Final Results of a Multicentre, Prospective, Observational Study', The Lancet, 393(10189), 2428-2438.
107 Prevention Access Campaign, Equal Access to the HIV Prevention Revolution, accessed 28 January 2020, available at <https://www.preventionaccess.org/>. >.
108 Eisinger RW, Dieffenbach CW, Fauci AS, 2019, 'HIV Viral Load and Transmissibility of HIV Infection: Undetectable Equals Untransmittable', JAMA, 321(5), 451-452.
109 De Francesco D, Sabin CA, Reiss P, 2019, 'Multimorbidity Patterns in People with HIV', Current opinion in HIV and AIDS, doi: 10.1097/COH.0000000000000595.
110 British HIV Association, 2008, UK National Guidelines for HIV Testing, London, British HIV Association, accessed 28 January 2020, available at <https://www.bhiva.org/file/RHNUJgIseDaML/GlinesHIVTest08.pdf>. >.
111 McCarthy GA, Mercey D, 1994, 'The Changing Clinical Features of HIV-1 Infection in the United Kingdom', Communicable Disease Report CDR review, 4(5), R53-R58.
112 Children's HIV Association, Enhancing the Health and Social Wellbeing of Children and Young People Living with HIV, CHIVA, Film, accessed 28 January 2020, available at <https://www.chiva.org.uk>. >.
113 Antiretroviral Therapy Cohort Collaboration, 2017, 'Survival of HIV-Positive Patients Starting Antiretroviral Therapy Between 1996 and 2013: A Collaborative Analysis of Cohort Studies', The Lancet, 4(8), HIV e349-e356.
114 Asad H, Sabin C, Collins I, et al., 2018, 'Severe Immunosuppression and Viral Failure in Adult Care Among Antiretroviral Therapy-Experienced Young People with Perinatal HIV in the UK', Reviews in Antiviral Therapy & Infectious Diseases, 8(19), 22, accessed 28 January 2020, available at <http://regist2.virology-education.com/abstractbook/2018/abstractbook_10ped.pdf>.>.
115 Asad H, Collins I, Goodall R, et al., 2018, 'Mortality and AIDS-Defining Events
Among Young People with Perinatal HIV Following Transition to Adult Care in the
UK', Reviews in Antiviral Therapy & Infectious Diseases, 8(27), 31, accessed 28 January 2020, available at <http://regist2.virology-education.com/abstractbook/2018/abstractbook_10ped.pdf>.>.
116 Foster C, Ayers S, McDonald S, et al., 2020, 'Clinical Outcomes Post Transition to Adult Services in Young Adults With Perinatally Acquired HIV Infection: Mortality, Retention in Care and Viral Suppression', AIDS, 34(2), 261-266.
117 Chhabra S, Fidler S, Bower M, et al., 2018, 'Malignancy and All-Cause Mortality: Incidence in Teenage Young Adults Living with Perinatally Acquired HIV', in International AIDS Conference, Amsterdam, 23–27 July, 7654.
118 Augustemak de Lima LR, Petroski EL, Moreno YMF, et al., 2018, 'Dyslipidemia, Chronic Inflammation, and Subclinical Atherosclerosis in Children and Adolescents Infected with HIV: The PositHIVe Health Study' PLOS ONE 13(1): e0190785, accessed 28 January 2020, available at <https://doi.org/10.1371/journal.pone.0190785>.>.
119 Concorde Coordinating Committee, 1994, 'Concorde: MRC/ANRS Randomised Double Blind Controlled Trial of Immediate and Deferred Zidovudine in Symptom Free HIV Infection', The Lancet, 343(8902), 871-881.
120 Darbyshire JH, Delta Co-ordinating Committee, 1996, 'Delta: Arandomised Double Blind Controlled Trial Comparing Combinations of Zidovudine Plus Didanosine or Zalcitabine with Zidovudine alone in HIV Infected Individuals', The Lancet, 348(9023), 283-291.
121 The Strategies for the Management of Antiretroviral Therapy (SMART) study Group, 2006, 'CD4+ Count-Guided Interruption of Antiretroviral Treatment', New England Journal of Medicine, 355(22), 2283-2296.
122 Walmsley SL, Antela A, Clumeck N, et al., 2013, 'Dolutegravir Plus Abacavir-lamivudine for the Treatment of HIV-1 Infection', New England Journal of Medicine, 369(19), 1807-1818.
123 The INSIGHT START Study Group, 2015, 'Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection', New England Journal of Medicine, 373(9), 795-780.
124 Larder BA, Kemp SD, 1989, 'Multiple Mutations in HIV-1 Reverse Transcriptase Confer High Level Resistance to Zidovudine', Science, 246(4934), 1155-1158.
125 Riddler SA, Haubrich R, DiRienzo AG, et al., 2008, 'Class Sparing Regimens for Initial Treatment of HV-1 Infection', New England Journal of Medicine, 358(20), 2095-2106.
126 Gueler A, Moser A, Calmy A, et al., 2017, 'Swiss HIV Cohort Study, Swiss National Cohort. Life Expectancy in HIV-Positive Persons in Switzerland: Matched Comparison with General Population', AIDS, 31(3), 427-436.
127 Antiretroviral Therapy Cohort Collaboration, 2008, 'Life Expectancy of Individuals on Combination Antiretroviral Therapy in High-Income Countries: A Collaborative Analysis of 14 Cohort Studies', The Lancet, 372(9635), 293-299.
128 Han WM, Apornpong T, Kerr SJ, et al., 2018, 'CD4/CD8 Ratio Normalization Rates and Low Ratio as Prognostic Marker for Non-AIDS Defining Events Among Long-term Virologically Suppressed People Living with HIV', AIDS Research Therapy, 15(1), 13.
129 Rockstroh JK, Peters L, Grint D, et al., 2013, 'Does Hepatitis C Viremia or Genotype Predict the Risk of Mortality in Individuals Co-Infected with HIV?', Journal of Hepatology, 59(2), 213-220.
130 Thornton AC, Jose S, Bhagani S, et al., 2017, 'Hepatitis B, Hepatitis C, and Mortality Among HIV-Positive Individuals', AIDS, 31(18), 2525–2532.
131 Rockstroh JK, Spengler U, Sudhop T, et al., 1996, 'Immunosuppression May Lead to Progression of Hepatitis C Virus-Associated Liver Disease in Hemophiliacs coinfected with HIV', American Journal of Gastroenterology, 91(12), 2563-2568.
132 Lo Re 3rd V, Kallan MJ, Tate JP, et al., 2014, 'Hepatic Decompensation in Antiretroviral-Treated Patients Co-Infected with HIV and Hepatitis C Virus Compared with Hepatitis C Virus-Monoinfected Patients: A Cohort Study', Annals of Internal Medicine, 160(6), 369-379.
133 European AIDS Clinical Society, 2014, Guidelines Version 7.1 November 2014, European AIDS Clinical Society, accessed 28 January 2020, available at <https://www.eacsociety.org/files/guidelines-7.1-english.pdf>. >.
134 Bischoff J, Mauss S, Cordes C, et al., 2018, 'Rates of Sustained Virological Response 12 Weeks After the Scheduled End of Direct-Acting Antiviral (DAA)-Based Hepatitis C Virus (HCV) Therapy from the National German HCV Registry: Does HIV Coinfection Impair the Response to DAA Combination Therapy?', HIV Med, 19(4), 299-307.
135 Jones P, Hamilton PJ, Bird G, et al., 1985, 'AIDS and Haemophilia: Morbidity and Mortality in a Well-Defined Population', British Medical Journal, 291, 695-699.
136 Miller R, Goldman E, Bor R, Kernoff P, 1989, 'Counselling Children and Adults About AIDS/HIV: The Ripple Effect on Haemophilia Care Settings', Counselling Psychology Quarterly, 2(1), 65-72.
137 Walker IR, Julian JA, 1998, 'Causes of Death in Canadians with Haemophilia 1980-1995', Haemophilia, 4(5), 714-720.
138 Darby I, Ewart DW, Giangrande IP, et al., 1996, 'Importance of Age at Infection with HIV-1 for Survival and Development of AIDS in UK Haemophilia Population', The Lancet, 347(9015), 1573-1579.
139 Ghirardini A, Puopolo M, Rossetti G, et al., 1995, 'Survival After AIDS Among Italian Haemophiliacs with HIV Infection. The Italian Group on Congenital Coagulopathies', AIDS, 9(12), 1351-1356.
140 Racoosin JA, Kessler CM, 1999, 'Bleeding Episodes in HIV-Positive Patients Taking HIV Protease Inhibitors: A Case Series', Haemophilia, 5(4), 266-269.
141 Zeichner BS, Harris A, Turner G, et al., 2013, 'An Acquired Factor VIII Inhibitor in a Patient with HIV and HCV: A Case Presentation and Literature Review', Case Reports Hematology, 628513, https://doi.org/10.1155/2013/628513..
142 UK Haemophilia Centre Doctors' Organisation, 2004, 'The Impact of HIV on Mortality Rates in the Complete UK Haemophilia Population', AIDS, 18(3), 525-533.
143 Avert, 2019, HIV Stigma and Discrimination, 10 October, accessed 28 January 2020, available at <https://www.avert.org/professionals/hiv-social-issues/stigma-discrimination#footnote1_0djr72g>.>.
144 British Association for Sexual Health and HIV, Clinical Effectiveness Group, 2015, 2015 BASHH CEG Guidelines on Tests for Sexually Transmitted Infections, accessed 28 January 2020, available at <https://www.bashhguidelines.org/media/1084/sti-testing-tables-2015-dec-update-4.pdf>.>.
145 Kessels RPC, 2003, 'Patients' Memory for Medical Information', Journal of The Royal Society of Medicine, 96, 219.
146 British HIV Association, 2018, Standards of Care for People Living with HIV, accessed 28 January 2020, available at <https://www.bhiva.org/file/KrfaFqLZRlBhg/BHIVA-Standards-of-Care-2018.pdf>. .pdf>.
147 British HIV Association, 2019, BHIVA Guidelines for the Management of HIV in Pregnancy and Postpartum 2018 (2019 second interim update), accessed 28 January 2020, available at <https://www.bhiva.org/file/5bfd30be95deb/BHIVA-guidelines-for-the-management-of-HIV-in-pregnancy.pdf>. >.
148 Give Blood, Health, Eligibility & Travel, Index H, accessed 28 January 2020, available at <https://my.blood.co.uk/knowledgebase/Index/H>. >.
149 NHS Blood and Transplant, Give Blood, Tests We Carry Out on Your Blood, accessed 28 January 2020, available at <https://www.blood.co.uk/the-donation-process/further-information/tests-we-carry-out/>.>.
150 Elwyn G, Barr PJ, Grande SW, 2015, 'Patients Recording Clinical Encounters: Path to Empowerment? Assessment by Mixed Methods', British Medical Journal Open, 5, e008566, doi: 10.1136/bmjopen-2015-008566.
151 HEP Drug Interactions, 2020, Interaction Checker, accessed 28 January 2020, available at <https://www.hep-druginteractions.org/>. >.
152 HIV Drug Interactions, 2020, Interaction Checker, accessed 28 January 2020, available at <https://www.hiv-druginteractions.org/>. >.
153 National Aids Trust, 2014, HIV Patient Information and NHS Confidentiality England, accessed 28 January 2020, available at <https://www.nat.org.uk/sites/default/files/publications/Jan-2014-HIV-Patient-Confidentiality-NHS.pdf>. >.
154 Realistic Medicine, What is Realistic Medicine is: and what it isn't, accessed 28 January 2020, available at <https://www.realisticmedicine.scot/about/>. >.
155 NHS, Shared decision making, accessed 28 January 2020, available at <https://www.england.nhs.uk/shared-decision-making/>. >.
156 Davenport MP, Khoury DS, Cromer D, et al., 2019, 'Functional Cure of HIV: The Scale of the Challenge', Nature Reviews Immunology, 19(1), 45-54.
157 Karimi-Shahanjarini A, Shakibazadeh E, Rashidian A, et al., 2019, 'Barriers and Facilitators to the Implementation of Doctor-Nurse Substitution Strategies in Primary Care: A Qualitative Evidence Synthesis', The Cochrane Database of Systematic Reviews, 4(4), CD010412.
158 Medical Research Council, Clinicals Trials Unit, UCL, 2017, RIVER: Towards an HIV Cure, Kick & Kill Approach, accessed 28 January 2020, available at <https://vimeo.com/181012372/cf8a1d19f6>.>.
159 Health and Safety Executive, Laundry Treatments at High and Low Temperatures, accessed 28 January 2020, available at <https://www.hse.gov.uk/biosafety/blood-borne-viruses/laundry-treatments.htm>. >.
160 Health and Safety Executive, 2003, Safe Working and the Prevention of Infection in Clinical Laboratories and Similar Facilities, accessed 28 January 2020, available at <https://www.hse.gov.uk/pubns/clinical-laboratories.pdf>. >.
161 Department of Health, 2002, Good Practice Guidelines for Renal Dialysis/Transplantation Units, accessed 28 January 2020, available at <https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/382207/good_practice_guidelines_renal_dialysis_transplantation.pdf>.>.
162 Health and Safety Executive, 2018, Managing Infection Risks when Handling the Deceased, accessed 28 January 2020, available at <https://www.hse.gov.uk/pubns/priced/hsg283.pdf>.>.
163 Human Fertilisation and Embryology Authority, 2018, Code of Practice, 9th Edition, Annex O: Storage of Gametes and Embryos, 204, accessed 28 January 2020, available at <https://www.hfea.gov.uk/media/2793/2019-01-03-code-of-practice-9th-edition-v2.pdf>.>.
164 Commission of Inquiry on the Blood System in Canada, 1997, The Krever Commission Report, Vol.1, accessed 28 January 2020, available at <http://epe.lac-bac.gc.ca/100/200/301/hcan-scan/commission_blood_final_rep-e/vol1-e.pdf>.
Content composed with the HTML code editor with live preview. Get a subscription to remove promotional messages or browse our directory for the best free online tools.
This addendum answers the remaining questions from the original and supplemental Letters of Instruction. The numbering below follows the paragraph numbering in those letters.
An explanation as to how HIV is transmitted (Point 14.4 and Supplementary Question 7)
Worldwide, the main routes of transmission for HIV are heterosexual sexual intercourse both from men to women and women to men (particularly in Sub-Saharan Africa) and vertical transmission (from mother to child). In the UK transmission between men who have sex with men (MSM) remains relatively the most common route of infection, but recent years have seen significant falls in transmission with earlier initiation of antiretroviral therapy reducing infectiousness and preventative measures (particularly including pre-exposure prophylaxis (PrEP)) becoming more widely available.
Figure 7: Number of new HIV infection annually by exposure group in UK 2009-2018 (Source PHE)
Injecting drug use, though now a relatively uncommon route of transmission, has been associated with an outbreak in Glasgow first recognised in 2015. Transmission through contaminated medical procedures is a relatively uncommon route for HIV transmission, but still occurs even in the setting of well managed health systems.
In general terms, the risk of HIV transmission depends on the interaction between a number of factors:
Individuals vary in their susceptibility to HIV infection. Most individuals can be infected with HIV, but not all. At one extreme, a small number of individuals (<1% of UK populations) carry two copies of a gene, part of which is missing (known as CCR5delta32 deletion or CCR5d32), rendering an individual effectively resistant to natural infection.
In one study of people living with haemophilia exposed to infected blood products, no CCR5d32 individuals became infected, even with high exposures.1 However, they only accounted for approximately 10% of the uninfected group and, by extension, other factors together are more important. It is likely that there are other genetic factors that might contribute to resistance, as well as differences in immune responses generated between individuals.
Viable HIV virus can be found both in semen and vaginal fluid. Different sexual acts are associated with different risks of transmission, usually expressed as the risk per sex act with a partner not on antiretroviral treatment. For example, the average risk of infection from receptive anal sex per sex act (1:90) is estimated to be higher than receptive vaginal sex (1:1000). The risks of condomless anal and vaginal sex are lower for the insertive partner than for the receptive partner.2
These risks are all but removed should the infected partner be on effective HIV treatment (with an undetectable viral load). Accumulating observational data linking viral load and transmission led to the "Swiss statement" in 20083 which was the first to state that unprotected intercourse carried negligible risk of HIV transmission in the setting of undetectable viral load (and no co-existing sexually transmitted infection).
Large randomised controlled trials both from Africa (HPTN052 trial in heterosexual couples which found 96% reduction in transmission4) and men who have sex with men in Europe (PARTNER study where no transmissions seen5) have strengthened the evidence to support the role of treatment in prevention.6 In recent years this has led to a campaign of awareness, particularly aimed at those living with HIV, of "U=U", or "Undetectable is untransmittable".
Prior to the institution of routine testing and treatment for HIV-positive mothers, transmission to infants was high, estimated in the UK to be 26.5% in 1993.7 HIV can infect the baby in the uterus, however in the absence of maternal treatment most transmissions occur at or after the time of delivery. Key to preventing transmission is for pregnant women to be aware of their HIV status as early as possible6, and at the latest by the end of the first trimester, and for those who are HIV positive to initiate effective antiretroviral therapy. Routine testing in pregnancy was introduced in England in 2000 and the UK in 2002. Uptake is now very high8 (over 99%). Early diagnosis allows introduction of effective antiretroviral treatment with time for the viral load to be fully suppressed which is key to preventing transmission.9 By 2011, the transmission of HIV from pregnant women to their babies had fallen to <1% in the UK.
HIV can be transmitted through breast feeding, although with the advent of HIV therapy and viral suppression the risk is low (estimated at 1.1% at six months if on effective treatment). In the UK and other high-income settings, the safest way to feed infants born to women with HIV is with formula milk, as there is no ongoing risk of HIV exposure after birth. The British HIV Association (2019) continues to recommend that women living with HIV feed their babies with formula milk. However, in low income settings given the risks attached to formula feeding and the benefits of breast milk in protecting from other life-threatening infections, breast feeding is still recommended.
Exposure to infected blood on unbroken skin is not believed to carry a risk of transmission. Exposure to mucous membranes (e.g. eye) is estimated to carry a risk of 1 in 1000 and a needle stick injury (skin puncture) of 1 in 300 if the infected individual is not on treatment. Risk factors for infection following accidental/occupational exposure are a deep injury with a hollow needle that has been in the vein or artery of an HIV-positive person who has late-stage disease and a high viral load.
Donated whole blood is manufactured into blood components and blood products for transfusion. Samples from every donation are tested to identify the donor's blood group and to screen for the presence of infection using tests for Hepatitis B, C, E, HIV, HTLV and syphilis. The results of these microbiological tests must be complete and found to be negative before the donation is released for transfusion.
In accordance with UK transfusion guidelines, samples from every donation are tested for HIV using two techniques: 1) serological tests to identify the presence of antibodies to HIV1 and HIV2 and presence of the HIV antigen p24 (antibody testing implemented by UK blood services in mid-1980s, combination testing in 2001) and 2) nucleic acid tests (NAT) for the presence of HIV RNA (implemented in 2003 following a single case of transfusion-related transmission where the look-back investigation demonstrated the implicated donation to be HIV NAT positive but serology negative). As above, these tests must be complete and found to be negative before a donation is released for transfusion. Any donations found to be reactive on these initial screening tests will undergo confirmatory testing. This is also discussed above at page 19.
Red cells collected from a single whole blood donation, commonly referred to as 'one unit' of red cells, are usually transfused to treat anaemia. Plasma from a single donation is frozen promptly as fresh frozen plasma (FFP) to preserve the concentration of clotting factors present and can be used to treat deficiencies of coagulation factors. Often a dose of three or four units of FFP will be indicated. FFP can be further processed into cryoprecipitate, a plasma fraction rich in fibrinogen, von Willebrand factor and Factor VIII. Cryoprecipitate was initially manufactured as a treatment for haemophilia A, but is primarily used now to replace fibrinogen, for example in bleeding patients. Cryoprecipitate from five individual donations is pooled for transfusion to adults, to allow a standard adult dose of ten donations to be transfused easily. Single donation units of cryoprecipitate are manufactured for small volume transfusion. One adult treatment dose of platelets, (also referred to as one unit of platelets), can be prepared in two ways; platelets from four whole blood donations may be pooled, or platelets may be collected from a single donor who has undergone a procedure called apheresis. Most platelet transfusions are used to treat or prevent bleeding in patients with haematological malignancies and those undergoing chemotherapy. All blood components are leucodepleted during manufacture to remove residual white cells. This reduces the risk of transmission of vCJD and CMV but not HIV, because infectious HIV virus particles are present in the plasma as well as infected lymphocytes of donations infected with HIV. As discussed above, all blood donations are tested for the presence of HIV and any HIV-infected donations would be identified and discarded.
Plasma derivatives (also known as blood products) differ from blood components in that they are manufactured from the pooled plasma of many (sometimes thousands of) donations, rather than a single donation. Examples of plasma derivatives include clotting factor concentrates, immunoglobulin (for example anti-D immunoglobulin), and albumin. Individual donations are subject to microbiological testing including HIV prior to pooling, and testing repeated on the pooled plasma. These large plasma pools undergo fractionation to collect the plasma constituent of interest, and undergo pathogen inactivation steps, for example solvent/detergent treatment (discussed further below) to reduce the risk of transfusion transmitted infection. Since 1999, plasma derivatives used in the UK have been imported as a vCJD risk reduction measure.
Historical data demonstrates the probability of HIV transmission following transfusion of one unit of infected blood product transfused to be of the order of 90%. No difference in rate of transmission is seen between cellular blood components and plasma components.
The risk of HIV transmission following blood transfusion has fallen over time (see figure 8) due to the implementation of strategies which aim to prevent infected donations entering the supply chain. These include the following (also discussed above at page 19):
Lipid enveloped viruses including HIV can be irreversibly inactivated by treating the large plasma pool with organic solvent and detergent which disrupt the lipid envelope. This process of viral inactivation cannot be applied to cellular blood components (red cell, platelets) because SD treatment would also disrupt the membrane of the cells intended for transfusion.
Methylene blue has a virucidal action and can be added to plasma to inactivate viruses present including HIV. The plasma plus methylene blue is exposed to visible light before the methylene blue is removed. This process can be performed on single units of FFP and cryoprecipitate.
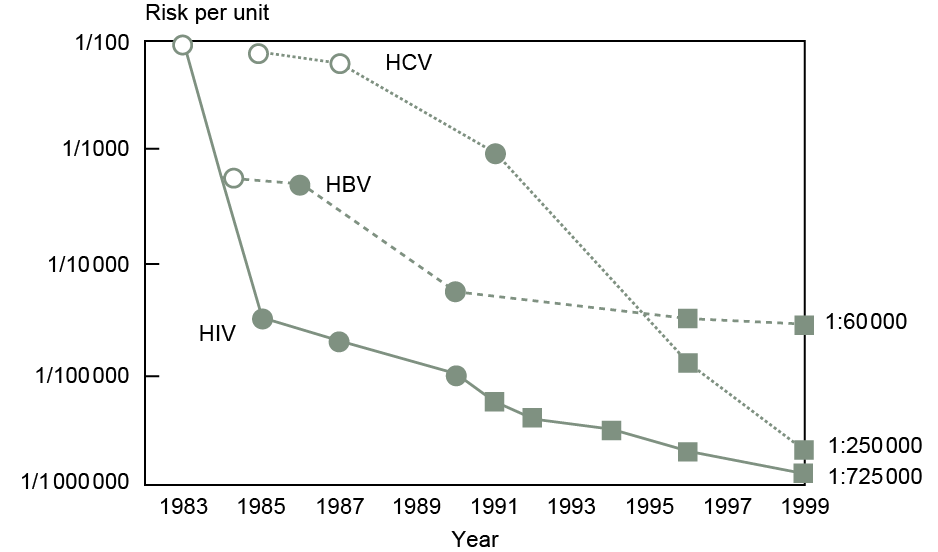
Figure 8: Estimated risk of transmission of HIV, HBV and HCV in the US over time. Solid circles are estimates based on recipient follow-up studies, open circles are estimates based on donor prevalence measurements and solid squares are projections based on mathematical modelling. Adapted from sources.11 and 12
As discussed above and at page 19, contemporary testing strategies employed by the blood services ensure the overwhelming majority of infected donations are identified and discarded. The small risk remains however that a donation with very low levels of virus present, for example in the very early stages of HIV infection, may pass these screening tests, leading to potential for transfusion of an infected component. In general, this risk is now low (estimated at 0.04 per million donations tested) as most seroconverting individuals have high viral loads which will be detected by antigen and NAT assays. This residual risk equates to one such infected donation being missed by screening tests every 15 years.10 The risk of transmission of infection is lower, because these calculations do not take into account the fact that some blood packs do not go on to be transfused, nor the susceptibility of a given patient to the infection or infectiousness of virus (discussed further in the next four sections).
Other scenarios which may result in HIV transmission by transfusion of infected blood include (i) technical failure of the tests (false negative) – this risk is reduced by inclusion of positive controls for tests (rare) (ii) a viral variant not detected by tests (rare).
In principle, one virion of viable HIV can establish infection in a susceptible individual. However, in general, the risk of transmission is expressed as the number of virions required to give a 50% probability of transmission. This number is conservatively estimated at 10. For a particular individual this value may be higher or lower.
The probability of different individuals being infected by the same contaminated batch will be affected by a range of factors including:
In a small study of individuals exposed to HIV-1-positive blood no clear differences were observed between those who became infected and those who did not.13
The risk of infection will, in general, increase with the amount of virus an individual is exposed to, so increased exposure to infected products will be associated with an increased risk of infection.
For an individual, there may be some (rare) exceptions, if an individual is highly resistant to infection.
It is possible for an individual already infected with HIV to be infected with an additional variant of HIV (so called "super infection"). Despite this, superinfection is generally uncommon.14 Superinfection may be associated with symptoms of acute infection (e.g. flu-like illness) and may be associated with a transient rise in an individual's HIV viral load. There is no evidence that superinfection per se leads to faster progression of HIV to AIDS in the absence of treatment.
What is the current optimum model of care for a person diagnosed with HIV? To include palliative care and how the care of co-infected people should be managed and coordinated (Supplementary Question 14)
The ways in which HIV remains apart from other clinical conditions influences models of care. HIV is a stigmatising, under-recognised, condition which disproportionately affects people who are already vulnerable and marginalised. There is still no cure for HIV and people live with a long term, potentially infectious, sexually transmissible and sometimes unpredictable condition. Unlike many other long-term clinical conditions HIV care is open access without the need for a GP referral and is free of charge for everybody in the UK irrespective of immigration status.
For people living with HIV, effective care translates into equality of access to person-centred health and social care that enables physical, psychological, emotional, and social well-being, promotes best health outcomes, and enhances quality of life.15The approach is based on achieving and maintaining maximal suppression of HIV activity using antiretroviral medication within a long-term condition model that gives appropriate attention to the prevention and treatment of HIV alongside associated co-morbidities. A multidisciplinary team approach is essential as well as collaborative working with a wide range of health and social care disciplines in both the voluntary and statutory sectors.
Models of care may differ to reflect local demographic and service patterns. Specialised clinical HIV services are most commonly delivered in outpatient settings through the specialties of sexual health, infectious diseases and clinical immunology. The British HIV Association Standards of Care 201815 set out the minimum care that a person living with HIV should expect to be able to access, along with auditable outcomes no matter where care is provided. These standards are considered the current UK benchmark and for them to be met requires services to work collaboratively within network structures, with no single service working in isolation.
HIV services have important interfaces with sexual health, reproductive health, mental health and drug and alcohol services. There is significant overlap with sexual health services both clinically and in terms of workforce. Although commissioned separately the same staff frequently work across both HIV and sexual health services which may impact on models of care for people living with HIV. People living with HIV have higher rates of psychological difficulties and mental health-related co-morbidities. Many HIV services have mental health professionals embedded within their teams which are integral to models of care. Reproductive decision making is an important yet complex part of living with HIV and easy access to appropriate advice care and support is essential for best outcomes.
The likelihood of long-term comorbidities alongside HIV is increasing particularly as people with HIV grow older, some of which are the inevitable consequence of old age and others are directly related to HIV and its treatment. As life expectancy has increased the broader HIV care agenda that is emerging needs whole system approaches focusing on integration of care across teams, disciplines and organisations. HIV services are becoming less self-contained. Close working relationships between HIV specialist services and primary care is particularly important. However current commissioning arrangements for the HIV pathway are complex and split across multiple commissioning bodies which can risk fragmentation of care.16 NHS England has a national service specification that is used as the basis for the commissioning of HIV treatment and care. A working group exploring long-term condition management for HIV has been established by NHS England.
The role of primary care within the HIV care model is variable. Although most people living with HIV are registered with a GP their role in the care pathway is often minimal. Outside the field of HIV, primary care teams have an important role in coordinating treatment and care across a range of conditions. In the context of HIV this is less well developed. Complex care needs of people with HIV may be coordinated within the specialist multidisciplinary HIV team, which may include clinical nurse specialists based in the community, rather than through primary care. Many general practices have only small numbers of patients with HIV and expertise may be less well developed. However, given the safety issues in polypharmacy, drug-drug interactions and co-morbidities clear and consistent communication between GPs and HIV services is particularly important for patient safety.
With the introduction of effective antiretroviral therapy fewer people develop the life-threatening immunosuppression related complications of HIV seen in the early years of the epidemic. This means that HIV services need to operate in networks across geographical areas to ensure adequate facilities, expertise and experience is available for people with advanced HIV-related complications requiring admission to hospital. Inpatient HIV services need to be able to provide 24-hour access for acute care that can be escalated to high dependency or intensive therapy units if need be. Specialist HIV physicians, nurses and pharmacists should deliver care with access to laboratory and other medical specialties and support services should be available on a 24-hour basis. Within networks there is a need for appropriate specialist input for complications associated with malignancies, co-infections and other end organ diseases. Services delivering HIV outpatient care must have documented agreed pathways to refer patients to specialist inpatient services as required. Specialist HIV inpatient services must be able to provide support and advice if patients cannot be physically transferred. Such support and advice should be available within 24 hours of request. Mechanisms should exist for seamless communication between inpatient and outpatient service to ensure effective discharge planning and arrangements for ongoing care.
In the early years of the HIV epidemic mortality rates from AIDS-related causes were very high and palliative care was an integral part of the care pathway. With the advent of effective antiretroviral therapy death rates from AIDS have fallen dramatically, there has been much greater focus on living with HIV and palliative care has received less attention. Today most deaths in the UK in people with HIV are due to non-AIDS-related conditions. Leading causes include liver disease, cardiovascular disease and cancers. Death due to AIDS is now most commonly seen in people who are diagnosed late in the course of HIV infection. People who have been living with HIV for many years and older people may have had multiple experiences of loss and ill-health among family friends and their wider communities. These experiences may mean that there are particular sensitivities relating to death and palliative care. From the point of diagnosis of a life-limiting condition, appropriate palliative care should form a constituent part of patient management based on a whole person approach that covers physical, psychological, social, and spiritual needs alongside pain and symptom control. Aspects of palliative care may be managed without the input of specialist teams however clear referral pathways should be in place between HIV and palliative care teams to ensure specialist expertise is available as required. Preferences for end-of-life care should be established, clearly documented and regularly reviewed. Clarity should be sought on the patient's preferences around sharing information, considering specific issues relating to HIV-associated stigma and confidentiality. A variety of person-centred outcome measures can be used to monitor symptoms and concerns.
TB, hepatitis B and hepatitis C are the most frequent co-infections occurring with HIV. All patients with HIV should expect to be screened for these three infections at the time of an initial diagnosis and again during follow-up in line with national guidelines. If diagnosed with a co-infection, people with HIV should have access to appropriate specialist care with clear arrangements for effective pathways of care and communication between different services. Communication between primary and secondary care services is essential to ensure patient safety as medication interactions in these situations may be particularly complex. People with co-infections should expect their treatment and care to be in line with the most up-to-date national guidelines.
To the extent that HIV and/or treatment for HIV causes or is linked to other health conditions (such as bleeding disorders) and secondary complications, to what extent should care for HIV and those conditions be coordinated, and what is current best practice and the optimum clinical model in this regard? (Supplementary Question 15)
As set out in the previous section about the optimum model of care, people living with HIV will also need care for other conditions which may or may not be related to HIV itself or its treatment. Best practice is to ensure that people living with HIV are at the centre of their own care, with the ability to have meaningful involvement in the development and delivery of services, as set out in the British HIV Association Standards of Care15. Many of the medications used to treat HIV can have significant interactions with medications for other conditions making it important that treatment information is shared in a seamless and timely way. Whenever there are multiple conditions requiring treatment and care, prioritising clear coordination between teams and specialties is crucial to ensure care is integrated and joined up. Care planning needs to address the interconnected needs of the individual patient rather than with the convenience of the separate elements of the healthcare system. The optimum clinical model in this regard is the one that delivers best outcomes. This will differ depending on demographics, case mix and the availability of different specialists. Joint clinics where specialists in both HIV and other disciplines are available to see the patient at the same time can be a useful model. In other situations, there may be regular discussions between specialists to review treatment and care plan. Best practice models ensure there is clarity about which providers are responsible for the management of each aspect of each condition and who is responsible for overall coordination of the care plan/care pathway.
Following successful treatment for HIV such that a person has an undetectable viral load, what follow-up scans, blood tests and/or checks and treatment should the person receive, how often and over what period of time? (Supplementary Question 18)
The British HIV Association (BHIVA) has published guidelines on monitoring of patients with HIV infection including those established on antiretroviral therapy (ART) with undetectable viral load.17 The guidance is similar to those published by other national and international HIV professional organisations.
Following initiation of ART, once patients have achieved viral suppression with an undetectable viral load and are clinically stable on treatment (usually after six months), they usually attend a specialist HIV clinical service every 6-12 months for review, depending on ART regimen, co-morbidities, adherence history and patient preference. The main purpose of each attendance is to review adherence to ART; factors that may adversely affect adherence (including mental health and welfare issues) adverse events related to ART (both clinical and laboratory); concomitant medication including potential drug-drug interactions; general physical and psychosocial wellbeing; and new symptoms and co-morbidities. Blood and urine tests are done to check that the viral load remains undetectable and to monitor for drug safety and other co-morbidities. CD4 count would usually be monitored if <350 cell/uL. Where appropriate a sexual health screen and checks for viral hepatitis A, B and C infection/immunity are recommended. Lifestyle factors such as smoking, and alcohol use are reviewed. For women, contraception needs, and pregnancy plans are reviewed and cervical screening for HPV infection undertaken at the recommended time interval. Other clinical investigations would be undertaken or onward referral to other specialties or primary care would be undertaken as clinically indicated.
People living with HIV infection are at an increased risk of ageing associated co-morbidities such as cardiovascular disease and cancers. Risk assessment for co-morbidities such as cardiovascular disease and bone fracture risk and prompt investigation of symptoms for early diagnosis of cancers are an important part of management and care of patients living with HIV.
Should the patient experience virological failure or problems with their antiretroviral regimen, then the regimen would be switched and monitoring increased till the patient is clinically stable and/or virological suppression has been re-established.
There is currently no cure for HIV infection, antiretroviral therapy and engagement with care and monitoring is lifelong.
What if any reporting and/or data collection methods are used to record secondary health conditions or complications which may have arisen from HIV/AIDS and/or any treatment received for the same? (Supplementary Question 20)
The UK surveillance and monitoring mechanisms record a variety of HIV related parameters.18 Information about new diagnoses of HIV, new diagnoses of AIDS and of death of people with HIV is collected from clinical services, laboratories, and other healthcare settings. In England, Wales, and Northern Ireland this data is collected and analysed by Public Health England (PHE), and in Scotland by Health Protection Scotland (HPS). Data is obtained from clinical providers on the use of HIV-specific services in England. This data is used to formulate national and local outputs such as the Public Health Outcomes Framework indicators and NHS England's HIV Quality Dashboard. However, there is fewer data that link secondary health conditions and HIV-related complications. Unlike many communicable diseases HIV is not a notifiable disease. This means there is no statutory/legal requirement for a case of HIV to be reported to PHE. Reporting of HIV-related data is undertaken by clinicians on a voluntary and consensual basis and is processed through the HIV and AIDS Reporting System (HARS). Information sent to PHE is 'de-identified' and the NHS number is not part of the dataset so potential linkages to other conditions are not made in the way they could be if the number were available.19
National surveillance of HIV infection and vertical HIV exposure is carried out by the National Surveillance of HIV in Pregnancy & Childhood (NSHPC) and covers all infants born to HIV positive women in the UK and Ireland, as well as all children diagnosed with HIV (regardless of country of birth) before the age of 16.20
There are several comprehensive research data sets that investigate secondary health conditions and complications. Public Health England has recently completed a nationally representative survey of patients attending HIV specialist care in England and Wales, Positive Voices. This has given rich data on secondary health conditions and complications as well as quality of life, social needs, and treatments. Currently this is running as a research project.21
The UK Collaborative HIV Cohort (UK CHIC) Study, established in 2001 collects data relating to clinical care and treatment of HIV. The study database contains routinely collected clinical information on HIV positive individuals aged over 16 years. Currently, the database contains more than 50,000 records of patients who have attended for care at one of a number of HIV clinics around the UK. The purpose of the study is to analyse the data to monitor the uptake and response to therapy among individuals with HIV in the UK. Other research themes include HIV and pregnancy; HIV and hepatitis co-infection; and HIV and ageing.22
In this response we have focussed only on medical training – we have not included nurse training or that of other healthcare professionals. Information has not always been easy to find as each speciality has its own training schedules and curricula.
Undergraduate medical students will usually learn about the basic science of HIV and AIDS relatively early in the curriculum, usually whilst studying microbiology, virology, and immunology. Training in the clinical aspects of HIV is more variable. Some medical schools deliver a course in Sexual Health Medicine of 2-3 weeks duration that includes opportunities to learn about HIV through tutorials, lectures, and clinic attendances. The basic science and immunology of HIV, diagnosis and natural history, acute presentations, opportunistic infections and malignancy, psychological aspects, treatment, hepatic, renal and neurological complications, PEP, prevention of mother-to-child transmission are usually covered. However, discussion with medical undergraduate students at various UK institutions23 would support the view expressed by the British Association for Sexual Health and HIV (BASHH) that "exposure to sexual health and HIV medicine is extremely varied between different medical schools with some only having a few days experience within the field. As a result, medical student's skills around sexual history taking and genito-urinary examination are equally variable".24
In one survey 28.6% of respondent medical students felt that they had "adequate knowledge" about HIV, while it was also stated that many medical students do not feel well informed about HIV.25 In another study 65% of final year medical students had taken a history from an HIV positive patient.26
In addition to formal undergraduate teaching other resources are available. For example, in an effort to supplement undergraduate medical teaching, BASHH provide an online "Toolkit for medical students"24 which aims to enhance knowledge and skills.
The management of HIV in children is regarded as a largely postgraduate issue. Paediatric textbooks do include a few broad paragraphs but little detail about HIV presentations and treatment in children. Medical students are unlikely to attend children's HIV clinics since the number of observers need to be limited and postgraduate trainees are given priority.
Internal Medicine Training (IMT) forms the first stage of specialty training for most doctors training in physicianly specialties. The diagnosis and treatment of HIV is included within Curriculum for Internal Medicine Stage 1 Training.27
The curriculum for General Practitioner training, "Being a General Practitioner" details the knowledge, skills and attitudes a trainee is required to achieve for certification.
Within the sexual health section of the clinical topics guide it states "HIV continues to be one of the most important communicable diseases in the UK. General Practice has a role in caring for patients with HIV and assessing the risk of having undiagnosed HIV. PrEP (Pre-Exposure Prophylaxis) is likely to become increasingly used to protect high risk individuals from becoming HIV positive."28,29
Several postgraduate deaneries are responsible for the provision and oversight of General Practitioner training around the United Kingdom.
Trainees will achieve competency and prepare for assessment by attendance at courses, clinical attachments, and the use of other learning resources.
Many postgraduate deaneries provide their own courses on sexual health problems.
In addition, trainees without a dedicated sexual health placement are encouraged to attend sessions at a relevant clinic.
Extensive material is available online and includes:
HIV is a small component of the curriculum for the training of General Paediatricians but is sufficient to sensitise UK-trained consultants to be aware of HIV in children. Paediatric trainees learn about the global epidemiology so that newly arrived children and young people are appropriately screened, and children with severe/unusual/persistent/recurrent infections are screened for immune deficiency which would include an HIV test. Prompt referral to specialists of any identified infected children is recommended.
The management of HIV in children is a sub-specialist discipline for which only Grid Trainees in Paediatric Infectious Diseases are expected to gain detailed knowledge. The accreditation of training in Paediatric Infectious Diseases has been agreed at European level through the European Society for Paediatric Infectious Diseases (ESPID). Attendance on an HIV training course has been a mandatory component, which is a blended online and residential course run by the Paediatric European Network for the Treatment of AIDS (PENTA). An abbreviated PENTA HIV module is also required for completion of the postgraduate diploma in Paediatric Infectious Diseases run by the University of Oxford.
Obstetrics and Gynaecology trainees must complete the core curriculum which covers aspects of sexual health and HIV.32
Health Education England (HEE) E-learning for Healthcare online eHIV-STI learning course is designed for medically trained postgraduates. The course was developed in collaboration with BASHH, the Royal Colleges of Physicians and e-Learning for Healthcare (e-LfH). This extensive e-Learning tool is relevant for clinicians in related specialties such as dermatology, infectious diseases, and gynaecology, as well as those in primary care wishing to increase their knowledge in sexual health and HIV.33
The British HIV Association Standards of Care15 state that ensuring healthcare workers have core knowledge about HIV is a key element in combating stigma within the NHS and it is recommended this teaching is included in the mandatory training undertaken by all employees. Ensuring this core HIV knowledge is also important for other staff working with vulnerable and/or affected groups including social care providers. However, despite this recommendation, such training is not universally available in health and social care settings.
References
1 Wilkinson DA, Operskalski EA, Busch MP, Mosley JW, Koup RA. A 32-bp deletion within the CCR5 locus protects against transmission of parenterally acquired human immunodeficiency virus but does not affect progression to AIDS-defining illness. The Journal of infectious diseases. 1998 Oct 1; 178(4): 1163-6.
2 Varghese B, Maher JE, Peterman TA, Branson B, Steketee RW. Reducing the Risk of Sexual HIV Transmission: Quantifying the Per-Act Risk for HIV on the Basis of Choice of Partner, Sex Act, and Condom Use, Sexually Transmitted Diseases 2002; 29: 38-43.
3 Vernazza P, Hirschel B, Bernasconi E, Flepp M. HIV infiziertre Menschen ohne andere STD sind unter wirksamer antiretroviraler Therapie sexuell nicht infektios. Bull Med Suisses 2008; 89: 165-69.
4 Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral Therapy for the Prevention of HIV-1 Transmission. The New England Journal of Medicine 2016; 375(9): 830-9.
5 Rodger AJ, Cambiano V, Bruun T, et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. The Lancet 2019; 393(10189): 2428-38.
6 Bavinton BR, Rodger, AJ. Undetectable viral load and HIV transmission dynamics on an individual and population level: where next in the global HIV response? Current Opinion in Infectious Diseases 2020; 33: 20-27.
7 Duong T, Ades AE, Gibb DM, Tookey PA, Masters J. Vertical transmission rates for HIV in the British Isles: estimates based on surveillance data. The BMJ 1999; 319(7219): 1227-9.
8 Public Health England. Progress towards ending the HIV epidemic in the United Kingdom 2018 report. 2019. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/821273/Progress_towards_ending_the_HIV_epidemic_in_the_UK.pdf (accessed May 2020) (accessed May 2020)
9 Hawkins D, Blott M, Clayden P, et al. Guidelines for the management of HIV infection in pregnant women and the prevention of mother-to-child transmission of HIV. HIV medicine 2005; 6(Suppl 2): 107-48.
10 Reynolds C, Davison KL, Brailsford SR. Safe supplies: few infections in UK blood and tissue donors. Transfusion medicine 2019; 29(4): 239-46.
11 Kleinman SH, Busch MP. The risks of transfusion-transmitted infection: direct estimation and mathematical modelling. Bailliere's best practice & research Clinical haematology 2000; 13(4): 631-49.
12 AuBuchon JP, Birkmeyer JD, Busch MP. Safety of the blood supply in the United States: opportunities and controversies. Annals of internal medicine 1997; 127(10): 904-9.
13 Busch MP, Operskalski EA, Mosley JW, et al. Factors influencing human immunodeficiency virus type 1 transmission by blood transfusion. Transfusion Safety Study Group. The Journal of Infectious Diseases 1996; 174(1): 26-33.
14 Diaz RS, Pardini R, Catroxo M, Operskalski EA, Mosley JW, Busch MP. HIV-1 superinfection is not a common event. Journal of Clinical Virology
15 British HIV Association: Standards of Care for People Living with HIV. 2018. Available at: https://www.bhiva.org/standards-of-care-2018 (accessed May 2020) (accessed May 2020)
16 Baylis A, Buck D, Anderson J, Jabbal J, Ross S. The King's Fund: The future of HIV services in England. 2017. Available at: https://www.kingsfund.org.uk/projects/future-hiv-services-england (accessed May 2020) (accessed May 2020)
17 BHIVA guidelines for the routine investigation and monitoring of adult HIV-1-positive individuals 2016 (2019 interim update). 2019. Available at: https://www.bhiva.org/monitoring-guidelines (accessed May 2020) (accessed May 2020)
18 Official Statistics. HIV: annual data tables. HIV surveillance data in the UK by demographic characteristics and geographical region. 2015. Available at: (https://www.gov.uk/government/statistics/hiv-annual-data-tables (accessed May 2020))
19 National AIDS Trust. HIV Patient Information and NHS Confidentiality. 2014. Available at: https://www.nat.org.uk/publication/hiv-patient-information-and-nhs-confidentiality (accessed May 2020) (accessed May 2020)
20 National Surveillance of HIV in Pregnancy and Childhood (NSHPC). Available at: https://www.ucl.ac.uk/nshpc/ (accessed May 2020) (accessed May 2020)
21 Public Health England. HIV: positive voices survey. Report presenting data from Positive Voices, the national survey of people living with HIV. 2020. Available at: https://www.gov.uk/government/publications/hiv-positive-voices-survey (accessed May 2020) (accessed May 2020)
22 The UK Collaborative HIV Cohort (UK CHIC) Study. 2001. Available at: http://www.ukchic.org.uk/overview (accessed May 2020) (accessed May 2020)
23 Personal communication (via David Johnson). 2020.
24 British Association for Sexual Health and HIV. Toolkit for medical students. Available at: https://www.bashh.org/events-education/toolkit-for-medical-students (accessed May 2020) (accessed May 2020)
25 National AIDS Trust. What do GPs learn about HIV? 2013. Available at: https://www.nat.org.uk/sites/default/files/publications/Dec_2013_HIV-GP-training.pdf (accessed May 2020) (accessed May 2020)
26 Jarrett, P, Sukthankar, A. Positive perspectives: an inter-university study of UK medical students on their attitudes to and knowledge of HIV. HIV Medicine 2013; 14(2): 12-13
27 Joint Royal Colleges of Physicians Training Board: Internal Medicine. Available at: https://www.jrcptb.org.uk/internal-medicine (accessed May 2020) (accessed May 2020)
28 Royal College of General Practitioners. Sexual health. Available at: https://www.rcgp.org.uk/training-exams/training/gp-curriculum-new/clinical-topic-guides/sexual-health.aspx (accessed May 2020) (accessed May 2020)
29 Royal College of General Practitioners. The RCGP Curriculum: Sexual Health. 2019. Available at: https://www.rcgp.org.uk/-/media/Files/GP-training-and-exams/Curriculum-2019/Curriculum-Topic-Guides-300819.ashx?la=en (accessed May 2020) (accessed May 2020)
30 Royal College of General Practitioners. eLearning resources. Available at: www.elearning.rcgp.org.uk (accessed May 2020) (accessed May 2020)
31 The Faculty of Sexual and Reproductive Healthcare: Education and Training. Available at: https://www.fsrh.org/education-and-training/ (accessed May 2020) (accessed May 2020)
32 Royal College of Obstetricians and Gynaecologists: specialty training core curriculum. Available at: https://www.rcog.org.uk/en/careers-training/specialty-training-curriculum/core-curriculum (accessed May 2020) (accessed May 2020)
33 Health Education England. e-Learning hub: sexual health and HIV. Available at: https://www.e-lfh.org.uk/programmes/sexual-health-and-hiv (accessed May 2020) (accessed May 2020)
Each contributing group member confirms that he or she understands his or her duty to provide independent evidence and has complied with that duty.
All contributing group members confirm that in respect of those parts of the report to which they have contributed:
Jane Anderson is chair of the National AIDS Trust and a past chair of the British HIV Association. She is a consultant physician in HIV medicine at Homerton University Hospital NHS Foundation Trust. She chairs the Public Health England Advisory Group for HIV and Sexual/Reproductive Health and she represents London clinicians in the NHS England Clinical Reference Group for HIV. She has worked as a clinician and researcher in HIV medicine since the virus emerged in the 1980s. She holds honorary academic appointments at St Bartholomew's Hospital, The London School of Medicine and Dentistry and University College London. Her work focuses on ethnic minority and migrant populations in relation to HIV in the UK, with a particular focus on HIV care for women and families. Her wide-ranging work engages with the current medical, social, ethical and legal challenges posed by HIV. She is also a visiting fellow at The King's Fund, an independent charity working to improve health and care in England.
Graham Cooke is a National Institute for Health Research (NIHR) Research Professor of Infectious Diseases at Imperial College London, Honorary Consultant and Lead for co-infection services within Imperial College NHS Trust. Previously, he was based at the Africa Health Research Institute in KwaZulu-Natal. He led the Commission on Accelerating the Elimination of Viral Hepatitis published in 2019, chairs the WHO Committee on the Selection and Use of Essential Medicines and is a member of the National Viral Hepatitis Strategy Group. He chairs the British HIV Association (BHIVA) Hepatitis Expert Advisory Group which is leading efforts for the microelimination of hepatitis C in those living with HIV. His current work focuses on precision medicine for managing infectious diseases and access to medicines, particularly for HIV/viral hepatitis. He led the clinical workstream for the MRC Stratified Medicine Consortium (STOPHCV) from 2003-19, and is infection lead for the London In-vitro diagnostics cooperative. He was chief investigator on the STOPHCV-1 trial and currently has studies running in the UK and Vietnam, in collaboration with the MRC Clinical Trials Unit.
Philippa Easterbrook is Senior Scientist in the Global Hepatitis Programme, HIV department at the WHO (World Health Organisation) Headquarters in Geneva. She is an HIV and infectious diseases physician and epidemiologist who has worked in the UK, United States and Sub-Saharan Africa. At WHO, she has led the development and dissemination of global normative guidance for HIV as well as Hepatitis B and C testing and treatment. She also provides technical guidance to national programmes worldwide on the implementation of hepatitis B and C testing and treatment scale-up programmes as part of a global elimination strategy. For eleven years, she was Head of Department, Professor of HIV Medicine, and consultant physician in Infectious Diseases at King's College London, and also Head of Research at the Infectious Diseases Institute in Uganda. Philippa has served as a Member of the UK Medical Research Council Infection and Immunity Committee, and was vice-chair of the World Health Organization Guidelines Review Committee. Her HIV research has encompassed epidemiology, clinical trials, operational and qualitative research, and laboratory-based studies.
Sian Edwards has worked as a nurse in HIV care for 32 years. Her experience includes both HIV clinical nursing and educational roles in HIV units in the UK, Australia and Zambia. She initially worked as an HIV community nurse in Sydney, Australia in 1986, a specialist lecturer in HIV and AIDS at St. Thomas' Hospital London and as a nurse lecturer at Chelsea and Westminster Hospital. Following two years working in Ndola, Zambia, in a Home-Based Care Program she returned to the UK as a community clinical nurse specialist in the Guy's and St Thomas' Haemophilia Reference Centre. Sian managed two research projects while a senior lecturer in HIV and Sexual Health at Brighton University, focusing on recording the life histories of people with haemophilia and HIV and their families. She has written on the topic of HIV care in nursing and management of AIDS, including a set of guidelines for healthcare workers in 1994. She is currently employed as an HIV Research Nurse Coordinator at Northside Clinic in Melbourne, and has just completed the life history project 'The AIDS Era: an oral history of UK healthcare workers.'
Professor David Goldberg is a consultant in Public Health Medicine and Clinical Epidemiology at Health Protection Scotland (HPS) who, over the last 25 years, has developed, implemented and evaluated interventions and monitoring techniques to prevent HIV and hepatitis B and C infections and their diseases, both nationally and internationally. He led the team which developed and coordinated the implementation of Scotland's Hepatitis C Action Plan. Previous roles include Henry Mechan Professor of Public Health, Deputy Director of Health Protection; and former Acting Director of the Scottish Centre for Infection and Environmental Health. He is an honorary professor of Public Health at the University of Glasgow and a professor of Public Health at Glasgow Caledonian University. He serves on several Scottish committees and is involved in the postgraduate supervision and teaching of students affiliated to the University of Glasgow, and is the author of approximately 250 peer-reviewed articles. He currently chairs Scotland's Hepatitis C Treatment and Therapies Group.
Dr Katie Hands is a Consultant Haematologist with the Scottish National Blood Transfusion Service (SNBTS) and is based at Ninewells Hospital in Dundee. She studied medicine at the University of Dundee, and during haematology specialty training undertook a PhD under the supervision of Professor Ron Hay. Dr Hands was appointed as a Consultant with SNBTS in 2016, providing transfusion medicine support for NHS Tayside, where her role includes promoting the safe and appropriate use of blood in all hospital departments. Her wider roles within SNBTS include policy development and transfusion medicine teaching as part of a National teaching programme for haematology registrars. She is a member of the British Society for Haematology Transfusion Task Force, and is involved in the preparation of evidenced based guidelines relating to all aspects of blood transfusion in the UK.
Dr Scott Jamieson is a General Practitioner in Kirriemuir, Scotland. He sits on the Royal College of General Practitioners (RCGP) Scottish Council, where he is the Executive Officer for Quality Improvement. He is Clinical Prescribing Lead for Angus Health and Social Care Partnership, sitting on the local Drug and Therapeutics Committee and the Non-Medicines Advisory Group. In addition to this, he is a GP trainer and GP representative on the Scottish Intercollegiate Guidelines Network (SIGN) Council, an organisation dedicated to improving the quality of health care for patients in Scotland by reducing variation in practice and outcome, through development and dissemination of national clinical guidelines. His interests include dermatology, minor surgery, and sexual and reproductive health.
Katie Jeffery is a consultant in Clinical Infection, the Infection Control Doctor (ICD) and Director of Infection Prevention and Control (DIPC) at the Oxford University Hospitals (OUH) NHS Foundation Trust. She trained in Microbiology with a particular interest in Virology, and has a PhD in HTLV-1 infection. Her clinical interests are infection prevention and control, viral diagnostics, viral hepatitis, and infections in the immunocompromised host. Previously she was Clinical Lead for Microbiology in the OUH, where she led on the introduction of a number of new laboratory assays in serology and molecular diagnostics. She holds a number of positions of responsibility both locally and nationally, including being a Virology Examiner for the Royal College of Pathologists, the Vice President of the British Infection Association, and a member of the Expert Advisory Group for Infectious Diseases for the MHRA (Medicines and Healthcare products Regulatory Agency). She has nearly 20 years' experience in treating patients with viral hepatitis, and is regularly involved as an investigator in national and international studies, especially those that benefit NHS patients by allowing early access to new drugs for the treatment of hepatitis C.
David J Johnston OBE is a General Practitioner at Maine Medical Practice in County Antrim. He is a Clinical Director of Dalriada Urgent Care, an out of hours primary care provider for the north east of Northern Ireland . He is also a member of "Practice 400" which provides care for General Medical Service designated "violent patients" and was involved in the establishment of ECHO, a scheme to enhance primary care services for homeless patients. His interest areas include pre-hospital immediate medical care, out of hours primary care and rural medicine. He has been a GlaxoWelcome research fellow with the University of Ulster and was involved in researching rural General Practice in Northern Ireland. David served as chairman of the NI Council of the Royal College of General Practitioners (RCGP) from 2008 to 2011 and was also a member of the UK council of RCGP. He continues to chair a number of community charities.
Jürgen Rockstroh is Professor of Medicine and Head of the HIV Outpatient Clinic at the University of Bonn, Germany, which treats the world's largest cohort of HIV-infected haemophiliacs. In addition to his clinical practice, Dr Rockstroh is involved in HIV research on: antiviral therapy, including new drug classes; the course of HIV disease in haemophiliacs; and HIV and hepatitis co-infection. He has been an investigator in multiple clinical trials of antiretroviral agents and treatments for HIV and hepatitis co-infection. He was the president of the German AIDS Society from 2007 to 2011, has been an executive committee member of the European AIDS Clinical Society (EACS) since 2009 and in 2019 was elected as president of EACS. Dr Rockstroh has been a member of the governing council of the International AIDS Society since 2011, and currently chairs the hepatitis research activities in NEAT (European AIDS treatment Network) and EuroSIDA. Between 2011 and 2017 he chaired the National German AIDS Advisory Panel, and the EACS co-infection guidelines. Dr Rockstroh has authored and co-authored over 500 publications in peer-reviewed journals, and over 70 book chapters. The German Society for Infectious Diseases awarded Dr Rockstroh the national AIDS research prize in 2005.
Dr Mallika Sekhar is a consultant haematologist and honorary senior lecturer at UCL. She specialises in myeloproliferative diseases and blood transfusion, across the University College London Hospital and Royal Free Hospitals, with a special interest in patients with vascular thrombosis and myeloproliferative diseases. She has been involved with writing Management Process Description (MPD) guidelines for the British Committee for the Standards in Haematology (BCSH). Dr Sekhar has been the Lead Investigator in studies on abdominal vein thrombosis in myeloproliferative diseases and transfusion in haematological malignancies, and a member of the National Cancer Research Institute (NCRI) MPD and supportive care clinical studies group. She was a member of the clinical expert panel on the Pathology Modernisation initiative for London, and is the lead for undergraduate education in haematology at the Royal Free campus. Previously, she was chair of the London Regional Council of the Royal College of Pathologists and has been a member of the National Blood Transfusion Committee Group on Education since 2012.
Professor Gareth Tudor-Williams is Professor of Paediatric Infectious Diseases at Imperial College London, and Consultant in Paediatric Infectious Diseases at Imperial College Healthcare NHS Trust, St. Mary's Hospital, London. His interest in paediatric infectious diseases was sparked after having worked for two years in the Himalayas for Save the Children Fund UK. He has spent 30 years contributing to some of the incremental improvements in the management of children living with HIV, and the prevention of vertical transmission from mothers to their infants. He is a Fellow at Duke University, North Carolina and a Visiting Scientist at the National Institutes of Health (NIH), Bethesda, Maryland. During his career, he has worked with the World Health Organisation (WHO) and UNICEF in the Northwest Sindh Province of Pakistan. Since 1994, he has helped run a multi-disciplinary service for children infected with HIV, young people and their families at St. Mary's Hospital. He was the founding chair of the Children's HIV Association of the UK and Ireland (CHIVA), alongside being a passionate undergraduate and postgraduate educator, for which he received the IC School of Medicine's Distinguished Teacher Award in 2017.
Dr Jonathan Wallis is a consultant haematologist. He has a keen interest in transfusion, and his publications include studies on leucodepletion and infection; transfusion-related acute lung injury (TRALI); long term survival after transfusion; and the 'Tag and Label' system for blood administration. Dr Wallis is an active member of regional and national transfusion committees, including having chaired the British Blood Transfusion Society (BBTS) Hospital Transfusion Special Interest Group. He also chaired the BBTS Scientific Meetings Administration Committee, and the International Society of Blood Transfusion (ISBT) Working Group on Clinical Transfusion. He initiated and runs the Newcastle course for Transfusion Practitioners and Biomedical Scientists on alternate years, and is also the Associate Editor of Transfusion Medicine.
Dr Ian Williams is a senior clinical academic in the Centre for Clinical Research in Infection and Sexual Health Institute for Global Health at University College London, and an honorary consultant physician at Central North West London NHS Trust and University College Hospitals NHS Trust. He has extensive clinical and research experience in HIV medicine. He has been involved with all aspects of care of HIV positive patients since 1987. His main research interests are antiretroviral therapy, HIV associated co-morbidities and primary HIV infection. He was chair of the British HIV Association (BHIVA) from 2008 to 2011 and has been a panel member of several national and international clinical guideline committees including chair of the BHIVA Antiretroviral Treatment (ART) guidelines in 2012. He was chair of the Clinical Reference Group for HIV (an expert advisory group) for NHS England from 2015 to 2019.